Published online Apr 14, 2006. doi: 10.3748/wjg.v12.i14.2254
Revised: August 1, 2005
Accepted: October 10, 2005
Published online: April 14, 2006
AIM: Many cirrhotic patients have muscular symptoms and rhabdomyolysis. However, myopathy associated with liver cirrhosis has not been established as a disease entity. We evaluated the clinical significance of acute myopathy associated with liver cirrhosis.
METHODS: We retrospectively reviewed the medical records of 5 440 cirrhotic patients who had been admitted to Gyeongsang National University Hospital from August 1997 to January 2003. Among these, 99 developed acute myopathies, and they were analyzed with respect to clinical and laboratory parameters, and outcomes.
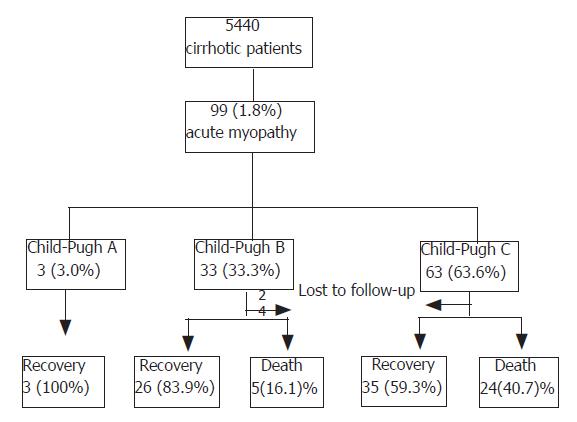
RESULTS: The Child-Pugh classification at the time of myopathy onset was A in 3(3.1%) cases, B in 33(33.3%), and C in 63 (63.6%). Infection was identified as the most predisposing factor to myopathy. Fifty percent of 18 idiopathic cases who were tested for influenza antibody were positive. Forty-two of the 99 cases were complicated by acute renal failure, and 25 (59.5%) of these expired. Apart from 6 cases lost to follow-up, 64 of 93 recovered, giving a mortality rate of 31.2%. Mortality was higher in Child-Pugh class C than in B or A.
CONCLUSION: Acute myopathy can develop as a serious complication in liver cirrhosis. Its frequency, severity and mortality depend on underlying liver function, and are higher in decompensated liver cirrhosis. Influenza should be considered as an etiologic factor in idiopathic cases. It is proposed that acute myopathy associated with liver cirrhosis be called ‘hepatic myopathy’, and that careful monitoring for hepatic myopathy is necessary in the patients with advanced liver cirrhosis.
- Citation: Lee OJ, Yoon JH, Lee EJ, Kim HJ, Kim TH. Acute myopathy associated with liver cirrhosis. World J Gastroenterol 2006; 12(14): 2254-2258
- URL: https://www.wjgnet.com/1007-9327/full/v12/i14/2254.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i14.2254
Since many investigators[1-3] had been interested in a causal relationship between alcohol ingestion and acute myonecrosis, Martin et al[4] systematically described the clinicopathological features of acute and chronic myopathy associated with alcoholism, and defined this as alcoholic myopathy. Now, it is well known that alcoholism and alcoholic liver diseases can be accompanied by alcoholic skeletal myopathy[5,6]. Moreover, a higher prevalence of muscle cramps was reported in patients with liver cirrhosis than in a matched population without cirrhosis, and it was suggested that muscle cramps be included as a recognized symptom of cirrhosis[7]. Muscular symptoms such as muscle cramps, weakness, aching and tenderness are also common in patients with liver cirrhosis, and even rhabdomyolysis can occur[8-10]. However, myopathy associated with liver cirrhosis has not been established as a disease entity. Here, we investigated the clinical characteristics of acute myopathy, which developed in patients with liver cirrhosis, and evaluated its clinical significance.
Of the 5 440 patients with liver cirrhosis who had been admitted because of various problems related to cirrhosis to Gyeongsang National University Hospital from August 1997 to January 2003, 99 patients who developed acute myopathy were included. We reviewed and analyzed their medical records retrospectively so to find out predisposing or etiologic factors, and possible factors influencing the frequency, severity, and prognosis of acute myopathy associated with liver cirrhosis.
A diagnosis of liver cirrhosis was made based on liver biopsy, or clinical, laboratory and radiological evidence of chronic hepatocellular dysfunction and portal hypertension. Underlying liver function was assessed by Child-Pugh’s classification based on laboratory data and physical signs at the time of most recent visit.
Acute myopathy was defined as an elevated serum muscle enzymes, i.e., creatine kinase (CK, 45-390 IU/L), lactate dehydrogenase (LDH, 30-170 IU/L), and aspartate transaminase (AST, 5-45 IU/L), and/or accompanied by acute muscular symptoms, such as muscle cramps, generalized or localized muscle weakness, muscle edema, muscle pain, or tenderness.
Possible predisposing factors were defined as the events occurring within one week prior to the onset time of myopathy.
Acute renal failure was diagnosed when the serum creatinine level increased by more than 50% of the baseline value, to above 1.5 mg/dL[11]. If there was no improvement in serum creatinine despite optimization of intravascular volume in azotemia, hepatorenal syndrome was diagnosed[12].
Exclusion criteria were a history of myocardiac infarction, cardiomyopathy, cerebrovascular accident and other cardiovascular diseases, thyroid disease, peripheral neuropathy, phlebitis, primary muscle disease, heat stroke, and underlying kidney disease, and the ingestion of agents known as triggering agents of myopathy such as digitalis, cimetidine, clofibrate, lithium, opiate, nifedipine, beta 2-agonist, beta-blockers, penicillamine (except for Wilson’s disease), cyclosporine, quinidine, antispastic drugs, verapamil, amphetamine, cocaine, vitamin E, or taurine[9,13].
Ninety-nine (1.8%) of 5440 patients with liver cirrhosis developed acute myopathy. With exception of 4 patients who developed myopathy during hospitalization after transarterial embolization, 95 patients were admitted due to acute myopathy. Seventy-four patients were male, giving a male to female ratio of 2.96. Mean age of patients was 51 ± 10 years (range; 27-76 years), and most were in the 6th decade. The etiology of the liver cirrhosis was alcohol in 47, hepatitis B virus in 41, hepatitis C virus in 5, Wilson’s disease in 1, and cryptogenic in 5 (Table 1).
| Clinical parameters | Number of cases (n=99) |
| Sex (M:F) | 74:25 (2.96:1) |
| Age (yr) range | 27 - 76 |
| mean | 51 ± 10 |
| Etiology of LC | |
| Alcohol | 47 |
| CVH (B) | 41 |
| CVH (C) | 5 |
| Wilson's disease | 1 |
| Idiopathic | 5 |
| Child-Pugh Class | |
| A:B:C | 3:33:63 |
Child-Pugh classification at the time of myopathy onset was A in 3 (3.0%), B in 33 (33.3%), and C in 63 patients (63.6%) (Table 1).
The most predisposing factor to myopathy was infection (46.5%); respiratory tract infections including common cold in 17, gastrointestinal infections in 12, urinary infection in 8, and septic shock in 5. Alcohol (8.1%), exercise or trauma (8.1%), transarterial embolization (4.0%), herb medicine (3.0%), dehydration (2.0%), and gastrointestinal bleeding (1.0%) were also possible predisposing factors of acute myopathy, and 27 cases (27.3%) were idiopathic (Table 2). In cases of transarterial embolization, acute myopathy developed after hepatic arterial embolization for primary liver cancer in two, and after splenic arterial embolization for splenomegaly with severe pancytopenia in two. Influenza antibody was checked in 18 of the 27 idiopathic cases and 9 (50%) had positive results.
| Predisposing factors | Number of cases (%) |
| Infection | 46 (46.5) |
| Respiratory infection | 17 |
| Gastrointestinal infection | 12 |
| Genitourinary infection | 8 |
| Septic shock | 5 |
| Alcohol | 8 (8.1) |
| Exercise or trauma | 8 (8.1) |
| Transarterial embolization | 4 (4.0) |
| Herb medicine | 3 (3.0) |
| Dehydration | 2 (2.0) |
| Gastrointestinal bleeding | 1 (1.0) |
| Idiopathic | 27 (27.3) |
Patients presented with muscle pain (57.6%) and/or generalized muscle weakness (23.2%). However, in 19 patients (19.2%) muscular symptoms were masked with concomitant complications, namely, hepatic encephalopathy (15.2%) or spontaneous bacterial peritonitis (4%).
Peak levels of AST, CK, and LDH in serum were 1264.8 IU/L, 20 693.2 IU/L, and 1926.7 IU/L, respectively. The intervals from symptom onset to peak levels of these muscle enzymes were 5.1, 5.4, and 6.2 days. According to Child-Pugh class, AST, CK, LDH levels were 925, 6 820.7, 1013 IU/L in A, 1 359.8, 21 083.3, 1 991.7 IU/L in B, and 1 231.2, 21 147.9, 1 936.1 IU/L in C, respectively. No statistically significant difference was found in muscle enzymes according to Child-Pugh class (Table 3).
Sixty cases received a urine orthotolidine test for myoglobin; 38 (63.3%) were negative, and 22 (37.6%) positive. The incidence of myoglobinuria was not different significantly between patients with or without renal failure (41.2% vs 33.4%). Serum myoglobin concentrations were high in all patients, but did not differ in myoglobinuria-negative and -positive patients (565 ± 267 vs 575 ± 183 mg/dL, P > 0.05), or between patients with or without renal failure (616.3 ± 332.1 vs 629.9 ± 269.8 mg/dL, P > 0.05).
A 99mTc-HDP (hydroxymethylene diphosphonate) radionuclide bone scan was performed in 28 patients, and radionuclide muscular uptake increased in 18 (64.3%) who received the test within a few days of onset. However, it should be noted that all 10 patients who did not show increased muscular uptake received the scan when they showed signs of symptomatic improvement rather immediately after onset.
Guidelines of discharge from hospital were the complete disappearance of muscular symptoms and a normalization or maintenance of muscle enzymes at < 1.5 times the normal value. Mean length of hospital stay was 20.8 ± 14.9 d, and this was longer for Child-Pugh B or C patients than for Child-Pugh A patients (21.6 ± 13.1 d, 20.4 ± 16 d vs 12.2 ± 3.1 d). Acute renal failure was a complication in 42 of the 99 (42.4%) acute myopathy patients.
Of the 93 patients followed, i.e., except the 6 lost to follow-up, 64 (68.8%) recovered, and 29 (31.2%) expired. In-hospital mortality was higher in Child-Pugh class C (24/59, 40.7%) than in Child-Pugh class B (5/31, 16.1%) or A (0/3, 0%)(Table 4, Figure 1). Forty of the 93 cases (43.0%) were complicated by acute renal failure, and 25 (62.5%) of these expired. The incidences of renal failure were 0%, 30.3%, and 49.2% in Child-Pugh A, B, and C, respectively. The most common causes of death were hepatic failure (14, 48.3%), renal failure (6, 20.7%), septic shock (6, 20.7%), and upper gastrointestinal bleeding (3, 10.3%).
Many investigators[7-9] have reported on the high prevalence of muscle cramps in patients with liver cirrhosis. Konikoff et al[7] found an 88% incidence of painful cramps in 33 cirrhosis patients, as compared to 21% in a matched population without liver disease. They concluded that the strikingly high incidence and uniformity of the phenomenon might justify the inclusion of painful muscle cramps among the recognized symptoms of cirrhosis. Abrams et al[8] suggested that cramps in cirrhotic patients are specifically related to the development of cirrhosis, and that a worsening liver function may be a risk factor for the development of cramps. Angeli et al[9] found that the prevalence of cramps was higher in cirrhotic patients than in controls, and that it was related to the duration of recognized cirrhosis and to the severity of liver function impairment. Moreover, they concluded that the pathophysiological link between cirrhosis and cramps may associate with a reduced effective circulating volume, and also indicated that weekly human albumin infusion may be an effective treatment for cramps in cirrhosis. However, these reports focused upon muscle cramps as a symptom observed in liver cirrhosis patients, and not on the clinical and laboratory findings associated with muscular symptoms. Moreover, in addition to muscular symptoms such as muscle cramps, weakness, aching and tenderness, rhabdomyolysis can also occur in liver cirrhosis patients[8-10]. However, no systematic investigation has been conducted on rhabdomyolysis development in cirrhotic patients.
Rhabdomyolysis may be defined as a clinical and laboratory syndrome resulting from skeletal muscle injury with the release of muscle cell contents into the plasma[14]. Increased concentrations of these released substances, such as CK, permit clinicians to diagnose this syndrome[14]. We found an incidence of acute myopathy of 1.8% among hospitalized liver cirrhosis patients. We investigated here cirrhotic patients with elevated muscle enzyme concentrations and/or muscular symptoms, i.e., rhabdomyolysis. And, we described this syndrome as acute myopathy rather than rhabdomyolysis, to better characterize the syndrome and to highlight its significance in liver cirrhosis patients, much like that of the myopathies of alcoholism and alcoholic liver diseases.
We observed that almost all cirrhotic patients who developed acute myopathy had a poor liver function, i.e., Child-Pugh B or C at the time of onset, with exception of 3 cases with Child-Pugh A. Therefore, patients with advanced liver cirrhosis and a poor liver function should be monitored carefully for acute myopathy, in addition to the better known complications of hepatic encephalopathy, gastrointestinal bleeding, or hepatorenal syndrome.
Compared with rhabdomyolysis in the general population, in which drug overdose and septicemia are the most common etiologic factors[15], we found that the most predisposing factor to myopathy development in cirrhotic patients was infection (46.5%), including respiratory, spontaneous bacterial peritonitis (E. coli), urinary tract infection, and Vibrio vulnificus sepsis. Furthermore, 50% of idiopathic cases were positive for anti-influenza antibody, suggesting that an infectious etiology may account for over 50% of acute myopathy associated with liver cirrhosis, and that influenza should be considered an etiologic factor of acute myopathy developing in cirrhotic patients without a definite etiology. In addition to influenza, various viral and bacterial infections have been reported to be etiologic factors of rhabdomyolysis in the general population[16-20]. Although the precise pathophysiology underlying virus or bacteria-induced rhabdomyolysis is unknown, proposed pathophysiological mechanisms include direct viral or bacterial invasion of skeletal muscle and toxin generation[17]. Muscle biopsies performed on rhabdomyolysis patients found lymphocytic infiltrate or viral inclusions, which support the hypothesis of direct viral invasion[21-23]. Viral DNA PCR analysis of muscle specimens and the identification of viral particles and bacteria in the muscle biopsy specimens, and the isolation of virus by culture from muscle specimens provide more compelling evidence of direct viral or bacterial invasion[22-24].
As 19.2% of our subjects presented with symptoms of concurrent complications other than muscular symptoms, acute myopathy may be difficult to identify in some cirrhotic patients by symptoms alone, i.e., without testing muscle enzymes. Because cirrhotic patients can develop various complications, muscle enzymes investigations are recommended for the detection of acute myopathy. On this point, previous investigations[7-9] were limited because they evaluated only muscular symptoms.
Increased levels of muscle enzymes are the result of skeletal muscle injury and the consequent release of muscle cell contents into the plasma[14]. AST, CK, and LDH peaked 5-6 d after symptom onset, and symptom alleviation was followed by a reduction in muscle enzyme levels (data not shown). The patients with decompensated liver cirrhosis had higher plasma levels of muscle enzymes than cirrhotic patients with a well preserved liver function. These findings represent the natural course of acute myopathy development in cirrhotic patients.
Rhabdomyolysis may or may not result in myoglobinuria, depending on the amount of myoglobin released into plasma, the glomerular filtration rate, and the urine concentration[14]. In the present study, myoglobinuria was present in only 37.6% of subjects who took the urine orthotolidine test; however, serum myoglobin was elevated in all patients. Moreover, myoglobinuria and serum myoglobin levels were unrelated to each other or to the development of renal failure. Different urine sampling times may also have influenced the low incidence of myoglobinuria. Though myoglobinuria is an important clue for the diagnosis of rhabdomyolysis, it cannot be used alone to identify all myopathies. Increased levels of muscle enzymes and serum myoglobin are more sensitive for the diagnosis of rhabdomyolysis, and in particular, CK is a highly sensitive marker of muscle injury[25].
Many case reports have mentioned the usefulness of Tecnetium-99 m bone scintigraphy for the early diagnosis, and for determining the location and extent of the muscle damage in rhabdomyolysis[26-28]. In the present study, a 99mTc-HDP radionuclide bone scan was diagnostic in 64.3% of the patients evaluated. Patients evaluated within a few days after onset showed increased muscular uptake, but not all patients showing symptomatic improvement who received this test showed increased muscular uptake. Thus, 99mTc bone agent scintigraphy may be useful for the evaluation of the degree and extent of muscle injury, if it is timely performed.
The length of hospital stay was about 12 d in patients with Child-Pugh A and about 3 wk in the patients with Child-Pugh B or C. Over 40% of cirrhotic cases with acute myopathy were accompanied by acute renal failure. The incidence of renal failure depended on Child-Pugh class, and was highest in patients with Child-Pugh C. Although the pathophysiologic mechanisms of rhabdomyolysis-related acute renal failure are unknown, several mechanisms have been proposed, for example, tubular obstruction by myoglobin plugs and/or urate, renal vasoconstriction caused by the inhibitory effect of myoglobin on endothelial vasodilator production, and toxic free radical produced by ferrous compound, a metabolite of myoglobin[29,30]. The frequency of acute renal failure in cases of rhabdomyolysis was reported to be 16%-33% in the general population[14,31], and 40.4% in acute myopathy associated with liver cirrhosis. In the present study, among rhabdomyolysis cases complicated by acute renal failure, 62.5% expired, and acute renal failure accounted for 20.7% of these mortalities. Thus, acute renal failure seems to have a prognostic role and its frequency was found to depend on Child-Pugh class. These findings suggest that the status of underlying liver function may be the most important prognostic factor in cases of acute myopathy associated with liver cirrhosis.
The mortality rate reported in the general population was 10%-12%[14,15,31], and the overall mortality rate in the present study was 31.2%, which is higher than those reported in cases of rhabdomyolysis in the general population. The mortality rate also depended on the status of underlying liver function. In particular, in-hospital mortality of Child-Pugh C patients was 40.7%. The most common cause of death in cirrhotic patients with acute myopathy was hepatic failure (48.3%).
There are some limitations in this study. First, since the present study was based on retrospective review of medical records of only symptomatic hospitalized patients, the incidence of acute myopathy was underestimated. In addition, all 5 440 cirrhotic patients were hospitalized because of various problems related to cirrhosis so that a majority of them had advanced diseases and the patients with well compensated diseases were not included. Therefore, for all that an analysis of all cirrhotic patients including those without any complication is necessary for defining the predisposing factors for acute myopathy, we could not compare underlying disease function between the patients with myopathy and those without. Thirdly, the diagnosis of myopathy was based on the laboratory and clinical findings, not electromyography or muscle biopsy. However, the decompensated cirrhotic patients can hardly receive electromyography or especially muscle biopsy. Furthermore, the term ‘myopathy’ was used as rhabdomyolysis in this study.
We found that acute myopathy development is a serious complication in liver cirrhosis patients and that this has several predisposing factors. Moreover, its frequency, morbidity, and mortality were found to depend on underlying liver function and to be higher in decompensated liver cirrhosis. The authors propose that acute myopathy associated with liver cirrhosis be called ‘hepatic myopathy’ and that careful monitoring is required for its early recognition in the advanced liver cirrhosis patients. The avoidance of predisposing factors by identifying disease mechanisms is certain to reduce the occurrence and mortality of hepatic myopathy. Further studies on the pathophysiologic mechanism and treatment of hepatic myopathy are warranted.
Footnote: Abstract of this paper was presented at Digestive Disease Week 2004, New Orleans, USA
S- Editor Wang J L- Editor Zhu LH E- Editor Cao L
| 1. | HED R, LARSSON H, WAHLGREN F. Acute myoglobinuria; report of a case with fatal outcome. Acta Med Scand. 1955;152:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | FAHLGREN H, HED R, LUNDMARK C. Myonecrosis and myoglobinuria in alcohol and barbiturate intoxication. Acta Med Scand. 1957;158:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Perkoff GT, Hardy P, Velez-Garcia E. Reversible acute muscular syndrome in chronic alcoholism. N Engl J Med. 1966;274:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Martin FC, Slavin G, Levi AJ. Alcoholic muscle disease. Br Med Bull. 1982;38:53-56. [PubMed] |
| 5. | Martin F, Ward K, Slavin G, Levi J, Peters TJ. Alcoholic skeletal myopathy, a clinical and pathological study. Q J Med. 1985;55:233-251. [PubMed] |
| 6. | Martin F, Peters TJ. Alcoholic muscle disease. Alcohol Alcohol. 1985;20:125-136. [PubMed] |
| 7. | Konikoff F, Theodor E. Painful muscle cramps. A symptom of liver cirrhosis. J Clin Gastroenterol. 1986;8:669-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Abrams GA, Concato J, Fallon MB. Muscle cramps in patients with cirrhosis. Am J Gastroenterol. 1996;91:1363-1366. [PubMed] |
| 9. | Angeli P, Albino G, Carraro P, Dalla Pria M, Merkel C, Caregaro L, De Bei E, Bortoluzzi A, Plebani M, Gatta A. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology. 1996;23:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Khokhar N. Massive rhabdomyolysis in cirrhosis of liver. J Pak Med Assoc. 2001;51:331-332. [PubMed] |
| 11. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1001] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 12. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1020] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 13. | Joekes AM. Cramp: a review. J R Soc Med. 1982;75:546-549. [PubMed] |
| 14. | Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore). 1982;61:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 582] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Black C, Jick H. Etiology and frequency of rhabdomyolysis. Pharmacotherapy. 2002;22:1524-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Cunningham E, Kohli R, Venuto RC. Influenza-associated myoglobinuric renal failure. JAMA. 1979;242:2428-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Singh U, Scheld WM. Infectious etiologies of rhabdomyolysis: three case reports and review. Clin Infect Dis. 1996;22:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Pesik NT, Otten EJ. Severe rhabdomyolysis following a viral illness: a case report and review of the literature. J Emerg Med. 1996;14:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Morton SE, Mathai M, Byrd RP, Fields CL, Roy TM. Influenza A pneumonia with rhabdomyolysis. South Med J. 2001;94:67-69. [PubMed] |
| 20. | Fernandez A, Justiniani FR. Massive rhabdomyolysis: a rare presentation of primary Vibrio vulnificus septicemia. Am J Med. 1990;89:535-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Foulkes W, Rees J, Sewry C. Influenza A and rhabdomyolysis. J Infect. 1990;21:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Porter CB, Hinthorn DR, Couchonnal G, Watanabe I, Caveny EA, Goldman B, Lash R, Holmes F, Liu C. Simultaneous Streptococcus and picornavirus infection. Muscle involvement in acute rhabdomyolysis. JAMA. 1981;245:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Pratt RD, Bradley JS, Loubert C, LaRocco A, McNeal RM, Newbury RO, Sawyer MH. Rhabdomyolysis associated with acute varicella infection. Clin Infect Dis. 1995;20:450-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Kessler HA, Trenholme GM, Harris AA, Levin S. Acute myopathy associated with influenza A/Texas/1/77 infection. Isolation of virus from a muscle biopsy specimen. JAMA. 1980;243:461-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Hess JW, MacDonald RP, Frederick RJ, Jones RN, Neely J, Gross D. Serum creatine phosphokinase (CPK) activity in disorders of heart and skeletal muscle. Ann Inter Med. 1964;61:1015-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Delpassand ES, Dhekne RD, Barron BJ, Moore WH. Evaluation of soft tissue injury by Tc-99m bone agent scintigraphy. Clin Nucl Med. 1991;16:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Yasuo M, Yamamoto H. [A case of rhabdomyolysis associated with influenza A viral infection given an useful early diagnosis by Tc-99m bone agent scintigraphy]. Kansenshogaku Zasshi. 2001;75:568-572. [PubMed] |
| 28. | Bhargava P, Bhutani C, Feng Q, Alavi A, Zhuang H. Varicella zoster infection associated rhabdomyolysis demonstrated by Tc-99m MDP imaging. Clin Nucl Med. 2003;28:594-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Abassi ZA, Hoffman A, Better OS. Acute renal failure complicating muscle crush injury. Semin Nephrol. 1998;18:558-565. [PubMed] |
| 30. | Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 245] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Kim HY, Choi SO, Shin SJ, et al. Analysis of 250 cases of rhabdomyolysis. Korean J Nephrology. 1994;13:810-817. |