Published online Apr 14, 2006. doi: 10.3748/wjg.v12.i14.2195
Revised: November 10, 2005
Accepted: December 23, 2005
Published online: April 14, 2006
AIM: To observe the effect of protocatechuic aldehyde on the proliferation of hepatic stellate cells (HSCs).
METHODS: Liver fibrosis was induced in rats by carbon tetrachloride (CCl4). Then normal and fibrotic drug sera were extracted from rats. The effects of protocatechuic aldehyde, raw Radix Salvia miltiorrhiza and drug sera of Salvia miltiorrhiza on HSC growth were determined by CCK-8. The protocatechuic aldehyde was separated by high performance liquid chromatography (HPLC) in a Alltima C18 column (250 mm × 4.6 mm, 5 μm) with a mobile phase of acetonitrile-4% glacial acetic acid solution (gradient elution) at the wavelength of 281 nm.
RESULTS: Protocatechuic aldehyde, raw Radix Salvia miltiorrhiza and drug sera of Salvia miltiorrhiza were found to have inhibitory effects on proliferation of rat HSCs. Raw Radix Salvia miltiorrhiza had a stronger inhibitory effect than the drug sera. The fibrotic drug sera showed a higher suppressive effect than the normal drug sera (P < 0.05). Protocatechuic aldehyde was found in crude materials of both Radix Salvia miltiorrhiza and its corresponding drug sera. The average recovery (n = 6) was 110.5% for raw Salvia miltiorrhiza Bge, 102% for normal drug sera and 105.2% for fibrotic drug sera. The relative standard deviation (RSD) was 0.37%, 1.96% and 1.51%, respectively (n = 6). The contents of protocatechuic aldehyde were 0.22%, 0.15% and 0.19%, respectively (n = 6) (P < 0.05). The RSD was 0.33%, 0.75% and 1.24% (n = 6) for raw material of Radix Salvia miltiorrhiza, normal drug sera and fibrotic drug sera, respectively. The samples were stable for 6 d.
CONCLUSION: Protocatechuic aldehyde can inhibit the growth of HSCs. HPLC is suitable for the determination of virtual bioactive components of Chinese herbal medicines in vitro.
- Citation: Lv T, Yao XX. Comparison of protocatechuic aldehyde in Radix Salvia miltiorrhiza and corresponding pharmacological sera from normal and fibrotic rats by high performance liquid chromatography. World J Gastroenterol 2006; 12(14): 2195-2200
- URL: https://www.wjgnet.com/1007-9327/full/v12/i14/2195.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i14.2195
Radix Salvia miltiorrhiza is an important traditional Chinese medicine (TCM) for activating blood and eliminating stasis[1]. Its effects on chronic hepatic diseases, such as liver fibrosis and cirrhosis, have been proved in experiments in vitro (cell culture) and in vivo (animal experiment)[2-4]. Liver fibrosis is a necessary stage during the development of liver cirrhosis. The activation and proliferation of hepatic stellate cells (HSCs) are the critical steps in hepatic fibrogenesis[5,6]. Radix Salvia miltiorrhiza could markedly inhibit the activation and proliferation of HSCs. Protocatechuic aldchyde is one of the bioactive components of Radix Salvia miltiorrhiza, which could inhibit the proliferation of HSCs and might be one of the efficient components of Radix Salvia miltiorrhiza acting on HSCs.
“Traditional seropharmacological method” usually utilizes healthy animals (such as rats) as medicine takers. The effect in vitro can be studied by isolating the drug sera (normal drug sera) from healthy animals to clarify the functions of the components in drug sera [7]. But different functional status of liver, which is the biggest organ for biological metabolism, could lead to some differences in the conversion of the same drug. Therefore, we used rats with liver fibrosis as the drug receivers and drug serum resources in our study. The corresponding drug sera (pathological drug sera) were drawn after oral administration of the herbs. We termed it “modified seropharmacological method”.
We compared the contents of protocatechuic aldchyde in raw Radix Salvia miltiorrhiza in normal and fibrosis rats before and after administration. Whether this method could predict the curative effects of Radix Salvia Miltiorrhiza was evaluated.
Twenty male Sprague-Dawley (SD) rats weighing 200-250 g were provided by the Laboratory Animal Center of Hebei Medical University. All rats were randomized into group A (normal rats) and group B (liver fibrotic rats), 10 in each group. The rats in group A were treated with saline, while the rats in group B were treated with 40% carbon tetrachloride (CCl4). Hepatic fibrosis was induced in rats of group B by 40% CCl4 4 mL/kg body weight for the first injection, then 2 mL/kg body weight, twice a week, for 9 wk.
HSC cell line was presented by Professor Greenwell, Marion Bessin Liver Research Center, Albert Einstein College of Medicine. The phenotype of CFSC was obtained from CCl4-cirrhotic liver of rats after spontaneous immortalization in culture.
RPMI-1640 was from GIBICO. CCl4 was of analytical grade. Radix Salvia miltiorrhiza was provided by Hebei Lerentang Chinese Medicine DrugStore Co. Drug sera were drawn from rats.
Radix Salvia miltiorrhiza was ground into powder and filtered with a sieve plate. The powder was dissolved into pure RPMI-1640 media to make the concentration 15 times that of the decoction for adults (within the range of safe concentration for HSCs). Then the dissolution was filtered to remove the bacteria for HSC cultivation.
SD rats weighing 200-250 g were randomized into group A and group B, 10 in each group. Liver fibrosis was induced in rats of group B by 40% CCl4, 4 mL/kg body weight first, then by 2 mL/kg body weight, twice a week, for 9 wk. The rats in group A were treated with saline. The decoction of Radix Salvia miltiorrhiza was administrated to all rats for 5 d. The quantity of raw Radix Salvia miltiorrhiza in solution was 1.33 g/mL. Blood was drawn from inferior vena cava 2 h after the drugs were given on the fifth day. Then drug sera were obtained by centrifugation at 3000 r/min for 20 min at 4 °C. The sera were deactivated at 56°C for 30 min. Then the drug sera were dissolved in RPMI-1640 to obtain 10% drug sera-1 640 medium for analysis.
Cell suspension (100 μl) was incubated on a 96-well plate. When HSCs grew to 90% confluence they were incubated in pure RPMI-1 640 overnight to synchronize HSCs into the G0 period. The solutions of protocatechuic aldchyde, raw Radix Salvia miltiorrhiza and the corresponding 10% normal and fibrotic drug sera-1 640 media in the wells were changed. The results were obtained. A group of controls was set at the same time. One hundred microliters of CCK-8 solution were added to each well of the plate after the drug sera were allowed to work for 24 h. Then the plate was incubated for 4 h. The absorbance (A) at 450 nm was measured with a microplate reader. The inhibitory rates (IR) of all solutions on the growth of HSCs were calculated (IR = experiment group-control group /control group×100%).
Waters 810 controller, Waters 486 tunable absorbance detector, Waters 510 HPLC pump, Millipore Waters U6K, Waters system interface module, Waters baseline 810 chromatography workstation (America) were used for HPLC. Sep-Park C18 purification column, ultrafree-MC filters (10 000 NMWL filter unit) and Autoscience ultrasonic producer were all from Millipore Corporation, America. Standard of protocatechuic aldchyde (NO. 110810-200205), glacial acetic acid and methanol were all special for HPLC. All reagents used in HPLC were filtered and deprived of vapor by ultrasound.
Radix Salvia miltiorrhiza (100 g) was ground into powder and filtered with a sieve plate. The powder of Radix Salvia miltiorrhiza was put into a volumetric flask containing methanol. Then ultrasound was used to facilitate the dissolution of the powder for 5 min and methanol was added to obtain 100 mL solution. This solution was then filtered through filters with 0.45 μm Millipore, purified by Sep-Park C18 column, and repeatedly filtered through Ultrafree-MC filters. Finally, the filtered solution was used as samples of raw Radix Salvia miltiorrhiza and kept in darkness at 4 °C.
The preparation of drug sera was the same as that of the sera for HSC cultivation. After the drug sera were obtained by centrifugation at 3000 r/min for 20 min at 4 °C, acetonitrile was mixed with the sera to get rid of protein by centrifugation. Then the supernatant was filtered through filters with 0.45 μm Millipore, purified by Sep-Park C18 column, and filtered through filters. Finally, the filtered solutions were kept in darkness at 4 °C.
The parameters for HPLC included: HPLC column: Alltima C18 column (250 mm × 4.6 mm, 5 μm); velocity of flow: 0.5 mL/min; detection wavelength: 281 nm; temperature for HPLC column: 30 °C; mobile phase: A: acetonitrile; B: 4% glacial acetic acid; gradient elution: A-B (2:98): 0-10min; A-B (10:90): 10-26 min.
Protocatechuic aldchyde (4.125 mg) was added into a volumetric flask. Then methanol was used to dissolve the powder to a final volume of 100mL (final concentration was 0.04125 mg/mL). Afterwards, 2, 5, 8, 10, 12, 15, 18 and 20 μL of protocatechuic aldchyde standard solution were injected into U6K for detection. Each volume was detected 3 times by HPLC under the conditions as described above. The standard curve was plotted with X axis (μg) to Y axis (mv.min).
For the experiment of exactitude, 10 μL of protocatechuic aldchyde standard solution (41.25 μg/ml) was injected into U6K for detection. The detection was repeated 6 times, 10 μL for each time. Then the relative standard deviation (RSD) of the peak areas was calculated to evaluate the exactitude of the experiment.
For the experiment of stability, the same volume of standard solution was injected into U6K for detection, 6 times each day for 9 d. The RSD of the peak areas each day was calculated to evaluate the stability of the samples during the 9 d.
In brief, 0.05 mg of protocatechuic aldchyde was put into 25 mg powder of raw Salvia miltiorrhiza. Then the samples for research were injected into U6K for detection. The average recovery rate (n = 6) was calculated.
Six samples of drug sera (400 μL for each sample) were taken out and 10 μL of protocatechuic aldchyde standard solution (0.04125 mg/mL) was dispensed into each sample of drug sera. Then the products were detected by HPLC (n = 6) to calculate the average recovery.
Radix Salvia miltiorrhiza (100 mg) was disposed as in preparation of samples of raw Radix Salvia miltiorrhiza. Then the samples were detected by HPLC for the content of protocatechuic aldchyde (n = 6). The detection was repeated 6 times independently. RSD of the peak areas was calculated to evaluate the repeatability of the experiment.
Four hundred µL of a certain drug serum was disposed as in preparation of samples of drug sera. The samples were detected for the content of protocatechuic aldchyde by HPLC (n = 6). RSD of the peak areas was calculated to evaluate the repeatability of the experiment.
The content of protocatechuic aldchyde in samples was detected by HPLC.
SPSS 10.0 was used to perform χ2 test and P <0.05 was considered statistically significant.
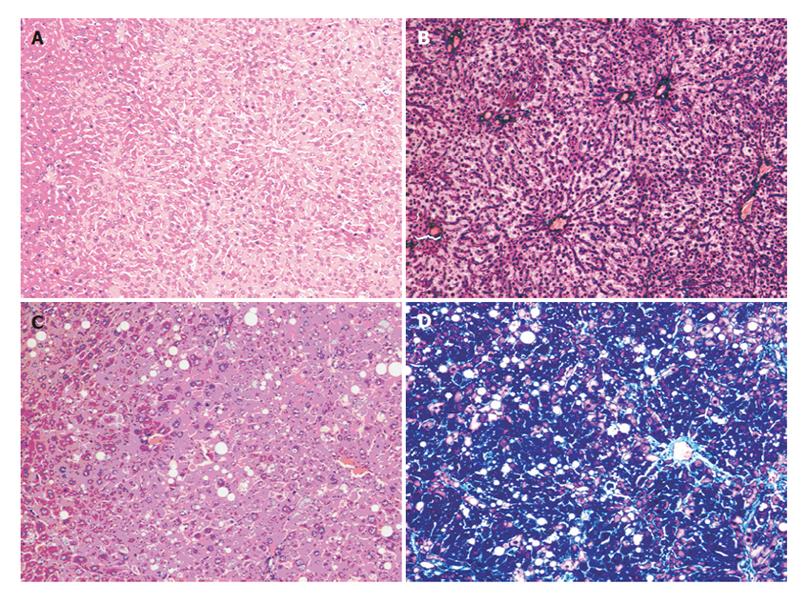
Obvious proliferation of fibers appeared in the livers when the rats in group B (Figures 1C and 1D) were treated with 40% CCl4 for 9 wk. There were obvious signs of vacuole degeneration, lobular inflammation and necrosis in the tissues of fibrotic liver. No evident changes appeared in the rat liver of group A (Figures 1A and 1B).
It showed that protocatechuic aldchyde, raw Radix Salvia miltiorrhiza, the corresponding normal and fibrotic drug sera could all inhibit the proliferation of HSCs compared with blank control group (P < 0.05). The raw Radix Salvia miltiorrhiza had a stronger inhibitory effect than the corresponding drug sera. The fibrotic drug sera of Salvia miltiorrhiza from fibrotic rats showed a higher suppressive effect on HSC growth than the normal drug sera from healthy rats (P <0.05) (Table 1). The contents of protocatechuic aldchyde showed a nice linearity within the range of 0.1-0.8 μg (Y=2.14×104X+2.77, r = 0.9999).
| Group | IR (%) | A |
| A | 24.36 | 1.565 ± 0.08a |
| (Protocatechuic aldchyde) | ||
| B | 36.10 | 1.322 ± 0.10a |
| (raw Radix Salvia miltiorrhiza) | ||
| C | 16.14 | 1.735 ± 0.12ab |
| (Normal drug sera of Salvia miltiorrhiza) | ||
| D | 21.41 | 1.626 ± 0.53ab |
| (Fibrotic drug sera of Salvia miltiorrhiza) | ||
| E | — | 2.069 ± 0.37 |
| (Control) | ||
Protocatechuic aldchyde standard solution was detected 6 times. The RSD was 0.33% for the peak areas of protocatechuic aldchyde. Within the 9 d of observation, the peak areas of protocatechuic aldchyde in standard solutions did not change significantly in the first 6 d. The RSD was 0.45% (n = 6) for the peak areas of protocatechuic aldchyde in the first 6 d. It indicated that the solutions of standards could be kept in darkness at 4 °C for 6 d.
The average recovery of raw Radix Salvia miltiorrhiza was 110.5%. RSD was 0.37% (n = 6). The average recovery of drug sera of Salvia miltiorrhiza was 102%, and the RSD was 1.96% (n = 6). The average recovery of fibrotic drug sera was 105.2%, and the RSD was 1.51% (n = 6).
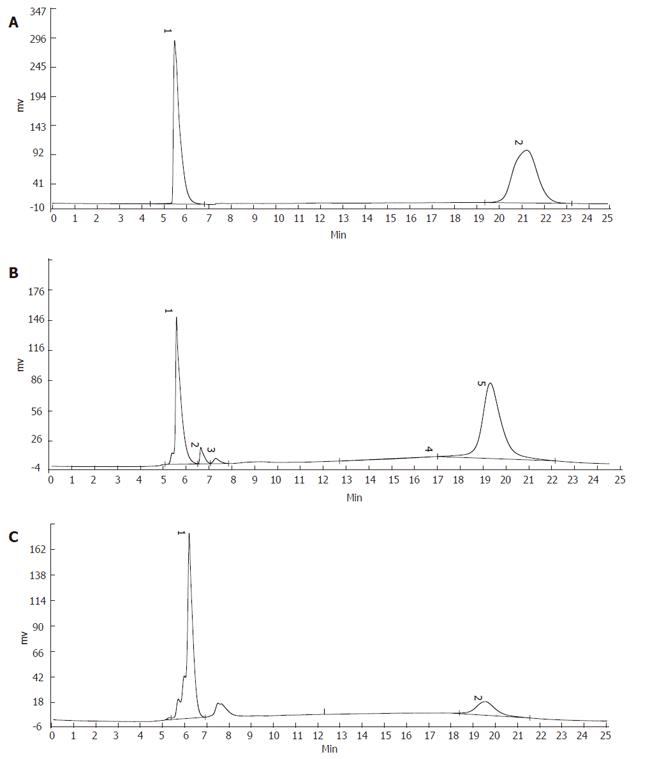
Protocatechuic aldchyde in raw Radix Salvia miltiorrhiza was 0.22%, and the RSD was 0.33% (n = 6). The ratio of protocatechuic aldchyde in normal drug sera was 0.15%, and the RSD was 0.75% (n = 6). The ratio of protocatechuic aldchyde in fibrotic drug sera was 0.19% and the RSD was 1.24% (Table 2, Figures 2A-2C).
Radix Salvia miltiorrhiza is the dried root of labiate-Salvia miltiorrhiza Bge, an important drug in traditional Chinese medicine for activating blood flow and eliminating stasis. Radix Salvia miltiorrhiza has certain curative effect on coronary heart diseases and chronic liver diseases with few adverse effects. No ideal anti-fibrosis drugs in Western medicine have been developed so far. A series of blood-activating and stasis-eliminating Chinese medicines such as Radix Salvia miltiorrhiza, have a positive effect on prevention and reversion of fibrogenesis. However, it is difficult to clarify the working mechanism and real active components of Chinese anti-fibrosis herbs. Radix Salvia miltiorrhiza can improve liver function and ameliorate hepatic pathological changes in rats and humans. In our study, when the aqueous extract of Radix Salvia miltiorrhiza was given to rats before the injection of CCl4, liver fibrosis was produced. On the other hand, in cell culture in vitro, Radix Salvia miltiorrhiza showed its inhibitory effect on lipid peroxidation and some key signal transduction circuits in HSCs, delaying the activation and proliferation of HSCs. A kind of extract from Radix Salvia miltiorrhiza-monomer IH764-3 could activate caspase-3 and induce apoptosis of HSCs.
Many bioactive components in Radix Salvia miltiorrhiza, including soluble and insoluble parts have various pharmacological effects such as blood-activating and stasis-eliminating and facilitate the circulation of blood and oxygen. The insoluble components mainly include compounds of phenanthrenequinone (PAQ), such as tanshinonesIand IIcryptotanshinone and isotanshinone. The soluble components are mainly composed of phenolic acids. Salvianolic acid B[8] is one of the bioactive parts of Radix Salvia miltiorrhiza to inhibit the activation and proliferation of HSCs by blocking the intracellular signal transduction of transforming growth factor-β1 ( TGF-β1 ).
Protocatechuic aldchyde is one of the water-soluble components of Radix Salvia miltiorrhiza and can be used to treat dysmenorrhea in gynaecology. Protocatechuic aldchyde can inhibit the proliferation of HSCs (CCK-8) in vitro and might be used to treat liver fibrosis. In this study, the peak areas maintained stable for 6 d. Since the stability of protocatechuic aldchyde is much better than other components (such as salvianolic acid B) in Radix Salvia miltiorrhiza, protocatechuic aldchyde performs the pharmacological effects for a longer time in vivo and in vivo.
The content of protocatechuic aldchyde in raw Radix Salvia miltiorrhiza is higher than that in the corresponding drug sera, which could explain the phenomenon that the inhibitory effect of raw Radix Salvia miltiorrhiza was stronger than that of drug sera. It indicates that only part of metabolites of protocatechuic aldchyde in raw Radix Salvia miltiorrhiza could enter blood to exert pharmacological effects after metabolism in vivo. If crude medicines are used to work on HSCs directly in vitro, the pharmacological effect of the medicine would be exaggerated. On the other hand, some other herbs could perform their pharmacological effect only after decocted in vitro and metabolized in vivo, because the process of decoction and metabolism would promote the activation of the bioactive components in this kind of crude medicines. Under this condition, the herbs seem useless if they are directly used on cells in vitro, suggesting that the effect of raw Radix Salvia miltiorrhiza is not equal to the effect of Salvia miltiorrhiza in vivo. The differences are related to the characteristics of herbs. Active constituents of TCM are influenced by soils, climates, growth stages, and factors in vitro and in vivo. So the “Pharmacological method” is more suitable for researching herbs.
Protocatechuic aldchyde in pathological drug sera is more than that in normal drug sera, which is in accordance with the different effects of normal and fibrotic drug sera [3]. Because of this difference between the two kinds of drug sera and the phenomenon that most of the medicine takers are patients with hepatic diseases, it is better to use “modified seropharmacological method” and “ pathological drug sera” to carry out research on herbs in vitro (vs“ traditional seropharmacological method” and “normal drug sera”). In this way, the impact of different liver functional status on herbs’ metabolic process could be included as a parameter in experiment. The phenomenon that the content of protocatechuic aldchyde is higher in fibrotic drug sera than in normal sera, coincides with the working characteristics of herbs, which might be caused by different “first pass effect” of the liver under different liver conditions and metabolism of drugs. From the traditional Chinese medicinal theory of “diagnosis and treatment on the basis of an overall analysis of the illness and the patient’s different symptoms and pathogenesis” and “bidirectional regulation”, TCM works best only when the symptoms and etiology are just their corresponding indications. Radix Salvia miltiorrhiza is one of the blood-activating and stasis-eliminating drugs. Liver fibrosis is considered as “blood obstruction”[9] in traditional Chinese medicine. Undoubtedly it is the indication for Radix Salvia miltiorrhiza. So the blood-activating and stasis-eliminating herbs such as Radix Salvia miltiorrhiza have better pharmacological effects on fibrotic rats. The active components in Radix Salvia miltiorrhiza might be activated and released more efficiently to perform stronger pharmacological effects. The ability of fibrotic liver to transform and inactivate drugs might decrease because the metabolic functions of fibrotic liver decrease, thus making the concentration of the effective components higher, prolonging the effective working period.
The value of this research lies in that we could get the real active components of Radix Salvia miltiorrhiza by “modified seropharmacological method” after they are metabolized in vivo. HPLC is a quick and sensitive method the determination of effective components in drugs in vitro and in vivo.
S- Editor Pan BR L- Editor Wang XL E- Editor Cao L
| 1. | Sun J, Huang SH, Tan BK, Whiteman M, Zhu YC, Wu YJ, Ng Y, Duan W, Zhu YZ. Effects of purified herbal extract of Salvia miltiorrhiza on ischemic rat myocardium after acute myocardial infarction. Life Sci. 2005;76:2849-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Nan JX, Park EJ, Kang HC, Park PH, Kim JY, Sohn DH. Anti-fibrotic effects of a hot-water extract from Salvia miltiorrhiza roots on liver fibrosis induced by biliary obstruction in rats. J Pharm Pharmacol. 2001;53:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Yao XX, Lv T. Effects of pharmacological serum from normal and liver fibrotic rats on HSCs. World J Gastroenterol. 2005;11:2444-2449. [PubMed] |
| 4. | She SF, Huang XZ, Tong GD. [Clinical study on treatment of liver fibrosis by different dosages of Salvia injection]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:17-20. [PubMed] |
| 5. | Vincent KJ, Jones E, Arthur MJ, Smart DE, Trim J, Wright MC, Mann DA. Regulation of E-box DNA binding during in vivo and in vitro activation of rat and human hepatic stellate cells. Gut. 2001;49:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Siegmund SV, Uchinami H, Osawa Y, Brenner DA, Schwabe RF. Anandamide induces necrosis in primary hepatic stellate cells. Hepatology. 2005;41:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Zhang QH, Zhong B, Chen KJ. [Effect of concentrated xuefu zhuyu pill on proliferation of vascular smooth muscle cells in experimental atheriosclerosis rabbits observed by serologic pharmacological test]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1996;16:156-159. [PubMed] |
| 8. | Liu C, Liu P, Hu Y, Zhu D. Effects of salvianolic acid-B on TGF-beta 1 stimulated hepatic stellate cell activation and its intracellular signaling. Zhonghua Yi Xue Za Zhi. 2002;82:1267-1272. [PubMed] |
| 9. | Yao XiX, Xu KC. Liver fibrosis-basis and clinic. Shanghai: Shanghai Scientific and Technological Education Publishing House 2003; 75-84. |