Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1949
Revised: August 2, 2005
Accepted: August 26, 2005
Published online: March 28, 2006
AIM: To characterize hyperlactatemia in patients with non-acetaminophen acute liver failure (ALF) in an attempt to clarify the mechanisms implicated and the role as a prognosis factor.
METHODS: In the setting of liver transplantation, 63 consecutive patients with non-acetaminophen acute liver failure were studied in relation to tissue oxygenation, hemodynamic and metabolic parameters. Before and after transplantation, the number of infected patients and outcome were registered.
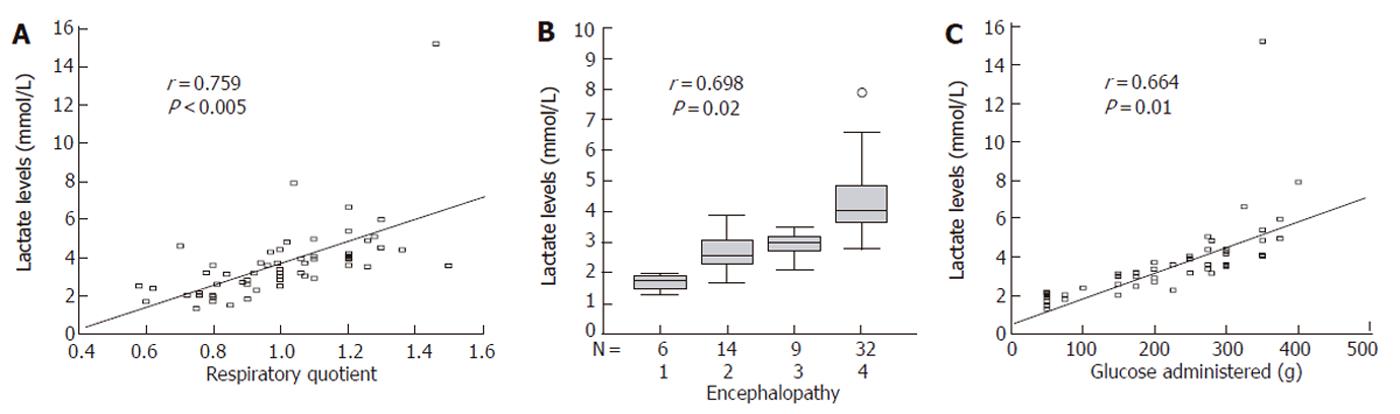
RESULTS: Acute ALF showed higher levels of lactate than subacute ALF (5.4 ± 1 mmol/L versus 2.2 ± 0.6 mmol/L, P = 0.01). Oxygenation parameters were within the normal range. Lactate levels showed good correlation with respiratory quotient (r = 0.759, P < 0.005), mean glucose administration (r = 0.664, P = 0.01) and encephalopathy (r = 0.698, P = 0.02), but not with splanchnic arteriovenous difference in PCO2, pH and the presence of infection (P = 0.1). Portal vein lactate was higher (P < 0.05) than arterial and mixed venous lactate, suggesting its production of hyperlactatemia in the intestine and spleen. The presence of infection was an independent predictor of survival.
CONCLUSION: Hyperlactatemia is not a prognosis factor due to byproduct of the overall acceleration in glycolysis.
- Citation: Taurá P, Martinez-Palli G, Martinez-Ocon J, Beltran J, Sanchez-Etayo G, Balust J, Anglada T, Mas A, Garcia-Valdecasas JC. Hyperlactatemia in patients with non-acetaminophen-related acute liver failure. World J Gastroenterol 2006; 12(12): 1949-1953
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1949.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1949
Up to now hyperlactatemia, a common finding in the setting of acute liver failure (ALF), has been attributed mainly to severe circulatory disturbance, abnormal vasomotor tone, plugging of small vessels by platelets and/or interstitial edema[1] and it has been proposed as a bad prognosis factor. Several studies suggest that splanchnic circulation abnormalities during ALF may result in inadequate distribution of blood flow and occlusion of the microvasculature, leading to tissue hypoperfusion, although oxygen delivery is increased in a macro circulation[2,3]. Inadequate balance between splanchnic oxygen delivery and demand results in splanchnic hypoxia of tissues that consume oxygen avidly[4]. In this condition, as cellular oxygen concentrations decrease, ATP concentrations cannot be maintained by oxidative phosphorylation. In an attempt to maintain cellular function, the cells shift over to anaerobic glycolysis with accumulation of lactic acid and hydrogen ions as ATP is hydrolyzed.
However, other mechanisms may be implicated in hyperlactatemia in the context of ALF. Although lactate is the end-product of anaerobic glycolysis and blood concentrations rise in response to hypoxia, well-oxygenated tissues may also produce lactate due to aerobic glycolysis[5]. On the other hand, since the liver is the principal organ responsible for removal of whole body lactate clearance, hyperlactatemia may be the result of a deficit of lactate clearance by the insufficient liver.
We undertook the present study to characterize hyperlactatemia in patients with non-acetaminophen ALF who were considered candidates for liver transplantation in an attempt to clarify the mechanisms implicated in high lactate levels as well as the role as a prognosis factor.
A total of 63 patients with diagnosis of non-acetaminophen ALF were admitted to the Liver Intensive Therapy Unit in our hospital and submitted to liver transplantation. All patients were prospectively evaluated and included in the study. Four of them were considered hyperacute, 39 acute while 20 subacute hepatic failure based on the criteria of the Kings College Hospital Group[6].
This prospective study was approved by the Clinic Hospital Research Ethic Committee. Informed consent for the study was obtained from each patient or patient’s family.
All patients were managed in the Liver Intensive Therapy Unit, in a standard manner[7]. They were maintained on 10%-20% glucose solution infusion (keeping blood glucose levels between 90 and 120 mg/dl). Prophylaxis for upper gastrointestinal bleeding and close microbiological surveillance (all patients received norfloxacin and nystatin) were performed. Coagulation profile was corrected only in the presence of overt bleeding. Those in grade III and IV coma (43 patients) were treated with standard sedation and mechanical ventilation. An epidural transducer (Ladd Research Laboratories, Burlington, VT) was inserted into the epidural space to enable early recognition and treatment of intracranial hypertension (>25 mmHg), mannitol (0.5 to 1 mg/kg) for over 30 min and pentobarbital infusion as a following step were used to control intracranial pressure (ICP). Systemic arterial hypotension (systolic <100 mmHg) was managed by dopamine infusion according to the hemodynamic conditions in an attempt to maintain cerebral perfusion pressure (CPP) (>60 mmHg). No artificial liver support techniques were used and no patient was treated with N-acetylcysteine or epoprostenol.
In 20 patients who remained awake (encephalopathy grades I and II), anesthesia was induced following our standard policy. All patients were mechanically ventilated (Servo 900C, Siemens) and the fraction of inspired oxygen was adjusted to achieve an arterial PaO2 of 180 - 220 mmHg. Ventilatory parameters were regulated to maintain the end tidal CO2 around 32 mmHg and PaCO2 below 35 mmHg. In all patients vasopressor dopamine at 3 µg/kg was started at the beginning of the surgical procedure and maintained through the transplant and increased to 8 µg/kg if necessary to achieve a mean arterial pressure greater than 75 mmHg. After graft reperfusion, arterial hypotension was treated by intravenous bolus of 10 μg of epinephrine. Fiberoptic pulmonary artery floatation catheter (7.5 French, Edwards Laboratories, Irvine, Calif.) was introduced through the right internal jugular vein and an arterial catheter (18 gauge, Arrow, Reading, PA) was placed via the left radial artery. At the beginning of the surgical procedure portal vein catheter (Certofix ® Mono S330 16G Braun) was placed by introducing it through a branch of the superior mesenteric vein and advanced to the portal vein until it was felt, in order to register portal pressure and to obtain blood samples for oxygen and metabolic parameters in splanchnic area. Not all patients received portal vein cannulation because it was not considered technically possible by the surgeon in 11 patients (4 with subacute ALF and 7 with acute ALF).
Before the surgical transplant procedure was started, the following parameters were measured. Mean arterial pressure (MAP, mmHg), cardiac index (CI, L/min/m2) and systemic vascular resistance index (SVRI, dyn.sec.cm-5.m2) were obtained.
Systemic parameters including oxygen content difference [D(a-v)O2, ml/dl], oxygen delivery (DO2, mL/min/m2), oxygen consumption (VO2, mL/min/m2), oxygen extraction ratio (VO2/DO2, %), mixed venous/arterial gradient of PCO2 (VACO2, mmHg), arterial/mixed venous gradient of pH (AVpH, U ) as well as respiratory quotient (RQ) were calculated.
Splanchnic parameters including arterial/portal venous oxygen content difference [D(a-v)O2, ml/dl], oxygen extraction ratio (VO2/DO2), portal veno/arterial gradient of PCO2 (VACO2, mmHg), and arterial/portal venous gradient of pH (AVpH, U) were obtained. All these variables were calculated following standard formulas.
Plasma lactate levels were measured with three blood samples simultaneously drawn from arterial catheter (La), the distal part of pulmonary catheter (Lv) and portal vein catheter (Lp). Blood lactate level was determined using a Kodak Ektachem 700XR (Rochester, NY. USA) analyzer[8].
The need of catecholamine administration and the mean glucose administration in the last 48 hours before transplant were recorded. The need of vasopressor drugs administered during the transplant procedure was also registered. The degree of encephalopathy and intracranial pressure were recorded.
Weight and structural characteristics of all explanted livers were studied.
Diagnosis of infection was made by the presence of the white blood cell count greater than 12×109/L or less than 4×109/L, the presence of immature neutrophils, temperature higher than 38 °C or lower than 36 °C and microbiological confirmation. Also, chest infection was confirmed by radiology. All these parameters were recorded daily during the ICU admission before transplantation and the ten following days. The number of infected patients and episodes and pathogens microbiologically confirmed was registered, respectively.
The mean stay in ICU during the first admission of patients after transplantation and immediate outcome (first admission in the hospital) were recorded.
Statistical analysis was performed using two-sided paired t-test for comparison of paired data and two-sided unpaired t-test for comparison of groups. The Bonferroni correction test was applied as appropriate. Categorical data were compared with the chi-square test for relationship between encephalopathy and infection. Correlation between lactate and the hemodynamic and oxygenation variables was assessed by linear regression analysis. Variables reaching significance in the univariate analysis between survivors and non-survivors were included in the multivariate analysis. Multivariate analysis was carried out by stepwise logistic regression analysis to determine discriminants of survival. All values were expressed as mean±SD. P < 0.05 was considered statistically significant.
Demographic and clinical characteristics of the patients are shown in Table 1. No patient needed blood transfusion before the transplant.
| Age (yr) | 32.7 ± 11 |
| Sex (M/F) | 26/37 |
| Etiology (n, %) | |
| Viral hepatitis | 18 (30) |
| Cryptogenic (non-A, non-B, non-C) | 32 (52) |
| Drug toxicity | 7 (11) |
| MAOi | 3 |
| Rifampin+Isoniacid | 2 |
| Isoflurane | 1 |
| α metil-dopa | 1 |
| Metabolic disease | 4 (6) |
| Encephalopathy | |
| I-II (subacute form: 20 p) | 20 (31.7) |
| III-IV (acute form:39 p) (hyperacute form: 4 p.) | 43 (68) |
Systemic hemodynamic parameters showed a hyperdynamic circulatory status with high CI (4.53 ± 1.4 L/min/m2) and low SVRI (1029±420 dyn.sec.cm-5.m2). Oxygenation parameters remained between normal ranges (DO2 623 ± 36 mL/min/m2, VO2 96.4 ± 21 mL/min/m2 and VO2/DO2 18.4 ± 3.1%).
The production of lactate in the intestine or in the spleen (Table 2), in accordance with Murphy et al[9], was suggested by the increased difference between portal, arterial and mixed venous lactate (P < 0.05). Acute ALF showed significant higher levels (acute: 5.4 ± 1 mmol/L, subacute: 2.2 ± 0.6 mmol/L, P = 0.01).
| Total(n = 52) | Acute(n = 36) | Subacute(n = 16) | P | |
| La (mmol/L) | 4.1 ± 1.8 (1.1-15.2) | 5.4 ± 1 | 2.2 ± 0.6 | 0.01 |
| Lv (mmol/L) | 4.3 ± 2 (1.4-16.3) | 4.7 ± 2 | 2.4 ± 1.4 | 0.03 |
| Lp (mmol/L) | 5.3 ± 1.1a (2.1-17.6) | 6.8 ± 1.8a | 2.9 ± 0.9 | <0.01 |
| pH (units) | 7.36 ± 0.07 (7.28-7.42) | 7.33 ± 0.02 | 7.36 ± 0.04 | NS |
| VACO2(mmHg) | 12.4 ± 7 (8.3-13.4) | 13.8 ± 5 | 9.6 ± 9 | NS |
| AvpH (units) | 0.06 ± 0.03 (0.02-0.07) | 0.08 ± 0.02 | 0.07 ± 0.04 | NS |
Lactate levels did not correlate with any of the hemodynamic or oxygenation parameters studied, except for the respiratory quotient (Figure 1 A). The grade of encephalopathy and the amount of glucose administered 48 hours before transplant (Figure 1 B and Figure 1 C) correlated well with blood lactate levels.
The splanchnic VACO2 and AVpH levels were in normal range and did not correlate with plasma lactate levels (r = 0.203 and r = 0.164, respectively). No differences were found (Table 2) when we compared patients with high (acute ALF) and low lactate levels (subacute ALF).
In order to maintain hemodynamic stability, only five patients with acute ALF needed administration of dopamine prior to the transplant. Only one of the patients demonstrated a high level of lactate (8.2 mmol/L). There was no significant correlation between lactate levels and the dosages of dopamine and epinephrine (r = 0.020 and r = 0.13 respectively) through the transplant. The total dose of epinephrine administered after graft reperfusion in patients with low (subacute ALF) and high lactate level (acute ALF) was similar in both groups (42.6 ± 12 µg and 54.1 ± 18 µg, respectively).
No relationship was found between lactate levels and liver weight. Interestingly, although the rate of massive necrosis was similar, liver weight of subacute ALF patients was significantly lower than that of acute ALF patients (762 ± 22 g and 932 ± 38 g, P < 0.05).
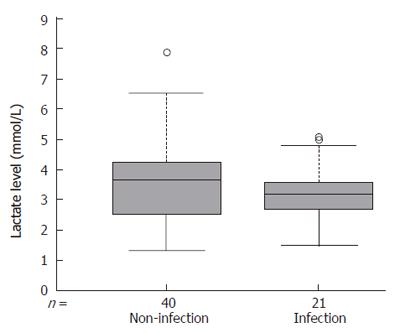
Twenty-one patients (33.3%) were infected in the perioperative period. Bacterial infection was found in 16 patients (25.4%), fungal infection in 5 patients (7.9%) and viral infection in 2 patients (3.1%). The incidence of infectious episodes of acute (13 patients, 30.2%) and subacute (8 patients, 40%) hepatic failure showed no difference. The level of lactic acidosis (Figure 2) no correlated with the presence of infection (P = 0.1). Sixteen patients were infected before the transplant, 11 of them requiring mechanical ventilation because of pulmonary infection (confirmed on chest radiograph and microbiologically), showed significantly higher arterial lactate levels compared with mixed venous lactate level (5.2 ± 1.1 and 4.5 ± 0.8 mmol/L, P = 0.03), suggesting lactate production within the lungs.
During the stay in ICU, 13 patients died (8 patients with acute and 5 subacute liver failure). Bleeding was not controlled in 2 patients. Furthermore, 3 patients needed retransplantation because the primary graft did not function and one of them died after retransplantation because of infection complications.
The mean stay in ICU was 14.6 days (range 4 - 38 d). The ICU stay correlated well with infection complications (P < 0.01) but not with plasma lactate levels. The mean plasma lactate level was similar in nonsurvivors and survivors (4.3 ± 0.7 mmol/L and 3.9 ± 10 mmol/L, respectively, (P = 0.69). Univariate analysis showed that infectious complications (71.4% vs 22.4%, P = 0.02) and subacute ALF (30% vs 18.6%, P = 0.08) were the potential risk of non-survivors compared to survivors. Multiple logistic regression revealed that the presence of infection was the only discriminator of survival. Encephalopathy grade and splanchnic VACO2 and AVpH did not significantly improve the prediction of immediate survival.
Hyperlactatemia observed in the context of non-acetaminophen ALF seemed to be related to aerobic glycolysis but not to tissue hypoperfusion. Additionally, the incidence of infection in these patients, which appeared as an independent predictor of survival, did not correlate with the degree of hyperlactatemia.
It is important to emphasize that the foremost cause of ALF in our series was acute viral hepatitis that differs substantially in other series. Since acetaminophen exerts a direct toxic effect on cellular respiration, this type of cytotoxicity may be different from other types of ALF.
Currently, the presence of hyperlactatemia in patients with ALF is assumed as the consequence of underlying overt tissue hypoxia. In these patients, oxygen delivery may be impaired. Combined measurement of VACO2 and AVpH may serve as a good indicator of tissue hypoxia with a closer relation to cardiac output than blood lactate, because it is less affected by changes in fuel substrate utilization or enzymatic alterations [10-12]. In our study no differences were found in splanchnic VACO2 and AVpH between patients with high (acute ALF) and low lactate levels (subacute ALF).
If hyperlactatemia is attributable to tissue hypoperfusion, the increase in oxygen delivery should reduce lactate levels. However, several reports[13-15] have failed to identify this evidence in patients with ALF due to acetaminophen overdose.
In our patients selected to receive liver transplantation, plasma lactate levels did not correlate with hemodynamic or oxygenation parameters, except for RQ and the amount of glucose administered, suggesting that aerobic glycolysis is responsible of lactate hyperproduction. Since patients with ALF may exhibit hyperinsulinemia due to pancreatic hypersecretion and/or decreased hepatic clearance of insulin, they need glucose infusion in order to maintain blood glucose level. Previous studies[16-18] showed that infusion of glucose results in a dose-dependent rise in splanchnic lactate levels. Moreover, peripheral tissues of cirrhotic patients produce an exaggerated lactate production in response to glucose administration[19]. In our study, 7 patients who did not require glucose administration (subacute liver failure), showed normal lactate levels (<1.3 mmol/L). A complete round of the Cori cycle is proton-neutral because the H+ produced by lactate from glucose is subsequently removed during synthesis of glucose from lactate in the liver. In the context of liver failure, the possibility to handle lactate to glucose (gluconeogenesis) is handicapped and consequently the possibility to develop “lactic acidosis” without the presence of high muscular lactate overproduction is not significant. In our patients the arterial blood pH stayed within normal ranges (7.28 to 7.41). If hyperlactatemia is not related to the mortality, infection, or operative hemodynamic management difficulty, the usage of dichloroacetate (which acts by stimulating pyruvate dehydrogenase) is not a priority unless its benefit to avoid postoperative severe alkalosis (lactate metabolism by the graft consume H+) is considered. However, it seems that this treatment fails to attenuate metabolic alkalosis after transplant[20].
Clemmesen et al[5] have suggested a hypermetabolic condition secondary to an excessively high glycolysis in relation to the small liver mass, but we have not shown any correlation between liver weight and splanchnic lactate level in patients with ALF. Wendon et al[21] demonstrated that there is a significant correlation between arterial lactate and survival in acetaminophen related ALF, but in our study no relationship was found between plasma lactate and outcome. Inadequate splanchnic blood flow and tissue hypoperfusion could contribute to bacterial translocation and sepsis, however in these patients several factors can predispose to infection such as comatose state, steroid therapy, mechanical ventilation and intravenous catheters. Patients with ALF are susceptible to infection as a result of multiple immunologic defects (excessive cytokine production from cells, such as monocytes and macrophages in response to a number of stimuli including bacterial lipopolysaccharide)[22]. In our patients the Gram-positive organisms were predominant. The presence of Gram-negative organisms such as pseudomonas was not uncommon, showing a high incidence in this group of patients. These results suggest that selective bowel decontamination can be used in the treatment of such patients[23]. We did not find any correlation between lactate levels and infection incidence although there is evidence that cytokines may promote augmented production of lactate[24] by several organs, enhancing cellular glucose uptake and glycolytic metabolism. However, in agreement with other studies[25], arterial lactate is higher than venous mixed lactate in patients with pulmonary infection.
In summary, the presence of high plasma lactate levels in patients with non-acetaminophen ALF, is not related to tissue hypoperfusion and is not a prognostic factor in the treatment of ALF.
S- Editor Guo SY L- Editor Wang XL E- Editor Bi L
| 1. | Makin AJ, Hughes RD, Williams R. Systemic and hepatic hemodynamic changes in acute liver injury. Am J Physiol. 1997;272:G617-G625. [PubMed] |
| 2. | Bihari D, Gimson AE, Lindridge J, Williams R. Lactic acidosis in fulminant hepatic failure. Some aspects of pathogenesis and prognosis. J Hepatol. 1985;1:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 71] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Bihari D, Gimson AE, Waterson M, Williams R. Tissue hypoxia during fulminant hepatic failure. Crit Care Med. 1985;13:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Clemmesen JO, Gerbes AL, Gülberg V, Hansen BA, Larsen FS, Skak C, Tygstrup N, Ott P. Hepatic blood flow and splanchnic oxygen consumption in patients with liver failure. Effect of high-volume plasmapheresis. Hepatology. 1999;29:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Clemmesen JO, Høy CE, Kondrup J, Ott P. Splanchnic metabolism of fuel substrates in acute liver failure. J Hepatol. 2000;33:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 412] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Castells A, Salmerón JM, Navasa M, Rimola A, Saló J, Andreu H, Mas A, Rodés J. Liver transplantation for acute liver failure: analysis of applicability. Gastroenterology. 1993;105:532-538. [PubMed] |
| 8. | Kropf J, Marx AM, Hildebrandt J, Gressner AM. Practical implications of coexistent different technologies in clinical chemical laboratories. Solid phase chemistry and conventional analysis. Eur J Clin Chem Clin Biochem. 1991;29:675-683. [PubMed] |
| 9. | Murphy ND, Kodakat SK, Wendon JA, Jooste CA, Muiesan P, Rela M, Heaton ND. Liver and intestinal lactate metabolism in patients with acute hepatic failure undergoing liver transplantation. Crit Care Med. 2001;29:2111-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Ducey JP, Lamiell JM, Gueller GE. Arterial-venous carbon dioxide tension difference during severe hemorrhage and resuscitation. Crit Care Med. 1992;20:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Bakker J, Vincent JL, Gris P, Leon M, Coffernils M, Kahn RJ. Veno-arterial carbon dioxide gradient in human septic shock. Chest. 1992;101:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Johnson BA, Weil MH. Redefining ischemia due to circulatory failure as dual defects of oxygen deficits and of carbon dioxide excesses. Crit Care Med. 1991;19:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 112] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 353] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Wendon JA, Harrison PM, Keays R, Gimson AE, Alexander GJ, Williams R. Effects of vasopressor agents and epoprostenol on systemic hemodynamics and oxygen transport in fulminant hepatic failure. Hepatology. 1992;15:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wendon JA, Harrison PM, Keays R, Gimson AE, Alexander G, Williams R. Arterial-venous pH differences and tissue hypoxia in patients with fulminant hepatic failure. Crit Care Med. 1991;19:1362-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Shulman GI, Lacy WW, Liljenquist JE, Keller U, Williams PE, Cherrington AD. Effect of glucose, independent of changes in insulin and glucagon secretion, on alanine metabolism in the conscious dog. J Clin Invest. 1980;65:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Myers SR, Biggers DW, Neal DW, Cherrington AD. Intraportal glucose delivery enhances the effects of hepatic glucose load on net hepatic glucose uptake in vivo. J Clin Invest. 1991;88:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Bratusch-Marrain PR, Waldhäusl WK, Gasić S, Korn A, Nowotny P. Oral glucose tolerance test: effect of different glucose loads on splanchnic carbohydrate and substrate metabolism in healthy man. Metabolism. 1980;29:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Leatherdale BA, Chase RA, Rogers J, Alberti KG, Davies P, Record CO. Forearm glucose uptake in cirrhosis and its relationship to glucose tolerance. Clin Sci (Lond). 1980;59:191-198. [PubMed] |
| 20. | Shangraw RE, Winter R, Hromco J, Robinson ST, Gallaher EJ. Amelioration of lactic acidosis with dichloroacetate during liver transplantation in humans. Anesthesiology. 1994;81:1127-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 23. | Salmerón JM, Titó L, Rimola A, Mas A, Navasa MA, Llach J, Ginès A, Ginès P, Arroyo V, Rodés J. Selective intestinal decontamination in the prevention of bacterial infection in patients with acute liver failure. J Hepatol. 1992;14:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Douzinas EE, Tsidemiadou PD, Pitaridis MT, Andrianakis I, Bobota-Chloraki A, Katsouyanni K, Sfyras D, Malagari K, Roussos C. The regional production of cytokines and lactate in sepsis-related multiple organ failure. Am J Respir Crit Care Med. 1997;155:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Routsi C, Bardouniotou H, Delivoria-Ioannidou V, Kazi D, Roussos C, Zakynthinos S. Pulmonary lactate release in patients with acute lung injury is not attributable to lung tissue hypoxia. Crit Care Med. 1999;27:2469-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |