Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1945
Revised: August 20, 2005
Accepted: August 24, 2005
Published online: March 28, 2006
AIM: To describe the clinical and histological characteristics of a group of adults with small-bowel nodular lymphoid hyperplasia (NLH).
METHODS: Patients were searched for five years in pathology records of our institution. The biopsy material was reassessed using strict histopathological criteria. Clinical data were obtained from medical records.
RESULTS: Small-bowel NLH was diagnosed in 18 cases. The female: male ratio was 2∶1. The most frequent symptoms were diarrhea (72%), involuntary weight loss (72%) and abdominal pain (61%). Nine patients (50%) had immunodeficiency. Small-bowel bacterial overgrowth was found in three (17%) cases. At small-bowel NLH diagnosis, three (17%) had associated lymphoma: two intestinal and one extra-intestinal lymphomas. In two patients with villous atrophy and anti-endomysial antibodies the diagnosis of celiac disease was established. Giardia lamblia infection was found in only one patient with hypogammaglobulinemia (Herman’s syndrome).
CONCLUSIONS: NLH is uncommon in adult patients. Associated diseases are immunodeficiency and lymphoid tissue malignancies.
- Citation: Rubio-Tapia A, Hernández-Calleros J, Trinidad-Hernández S, Uscanga L. Clinical characteristics of a group of adults with nodular lymphoid hyperplasia: A single center experience. World J Gastroenterol 2006; 12(12): 1945-1948
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1945
Nodular lymphoid hyperplasia (NLH) of the gastrointestinal (G1) tract is characterized by markedly hyperplasic, mitotically active germinal centers with well-defined lymphocyte mantles[1]. The nodules are found in mucosa and/or submucosa anywhere in the gastrointestinal tract and may resemble small adenomatous polyps on gross examination[2,3]. Approximately 20% of adults with common variable immunodeficiency (CVI) are found to have NLH. It has been suggested that NLH is a risk factor for both intestinal and extra intestinal lymphoma[4]. It has also been reported in patients with human immunodeficiency virus infection[5]. In children, NLH tends to have a benign course and usually regresses spontaneously but in adults it is rare and poorly described.
In a previous report by Canto et al[6] NLH was diagnosed in 11 Mexican subjects. In five of them a diagnosis of Herman’s syndrome was established. Patients were collected from the pathology database of our institute from 1973 to 1988. Since this time, an increased number of duodenal biopsies have been taken for the approach of patients with chronic diarrhea who were referred to our institution from different places. In this work we described the clinical characteristics of 18 new patients with NLH seen at the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, a National Institute of Health located in Mexico City, which is a referral center for gastrointestinal diseases.
From January 1998 to December 2002, patients with the histological diagnosis of NLH were identified from the pathology database. Medical records were reviewed and a pathologist (STH) reassessed slides or paraffin-embedded biopsies. NLH was considered when the following histological criteria were observed: hyperplasic lymphoid follicles, mitotically active germinal centers with well-defined lymphocytes mantles, and lymphoid follicles localized at mucosa and/or submucosa.
The original duodenal mucosal biopsy specimens were used for reassessment. The histopathological evaluation was performed on hematoxylin-eosin-stained samples, including counting and localization of lymphoid follicles, type and severity of inflammation in lamina propria, and presence of villous atrophy. If the quality of the original sections was too poor for the reassessment, new sections from the original paraffin wax-embedded biopsy blocks were made.
Age, duration and type of symptoms, body mass index (BMI) and associated diseases were evaluated. The following tests were recorded at the time of diagnosis: hemoglobin, mean cell volume, total proteins, albumin, globulins, β-carotene, electrolyte profile, prothrombin time, partial thromboplastin time, human immunodeficiency virus antibody, liver enzymes, stool analysis for ova and parasites, microscopic examination and culture of sterile collected duodenal fluid.
Clinical and histopathological data were expressed as absolute and/or relative frequencies and mean±SD. The Mann-Whitney U or χ2 tests were used to compare groups. P < 0.05 was considered statistically significant. SPSS software (version 10) was used.
Eighteen out of 550 duodenal biopsies had the histological diagnosis of NLH. After re-evaluation of the histological material all of them were included in the analysis. Twelve were women and 6 were men, yielding a female: male ratio of 2:1. Their median age at diagnosis was 41 years (18 to 76 years). Duration of symptoms from the onset to diagnosis varied from 1 to 48 mo (median = 18 mo). The median body mass index at diagnosis was 19 kg/m2 (11 to 26 kg/m2).
Diarrhea and weight loss were the predominant symptoms in 13 (72%) patients. A mean of 6 ± 4 bowel movements per day and a weight loss of 11.6 ± 6.9 kg in the last six months were presented. Eleven (61%) had abdominal pain (Table 1).
| Symptom or sign | n (%) |
| Diarrhea | 13 (72) |
| Involuntary weight loss | 13 (72) |
| Abdominal pain | 11 (61) |
| Lymphadenopathy | 4 (22) |
| Pallor | 4 (22) |
| Chronic cough | 2 (11) |
| Edema | 2 (11) |
| Arthralgia | 1 (6) |
| Neuropathy | 1 (6) |
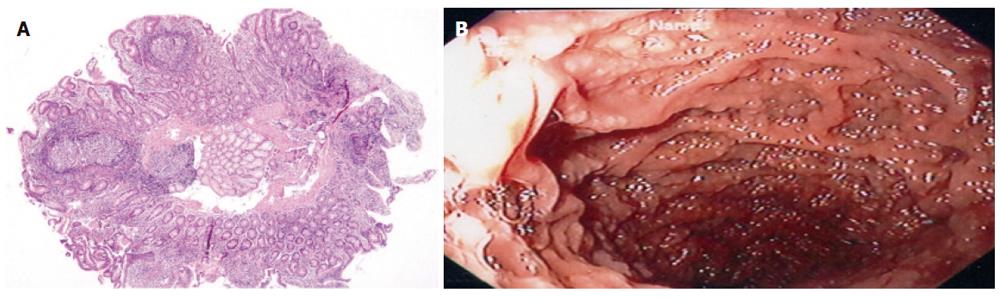
A mean of 2.5 (range 2-6) hyperplasic lymphoid follicles was observed in a single field 4 × magnification with light microscopy. Hyperplasic lymphoid follicles were localized in mucosa of 13 cases (69%) and in mucosa and submucosa of five cases (31%) (Figure 1).
Patients with 3 or more hyperplasic follicles had lower β-carotene levels (46.7 ± 25 mg/dL vs 108 ± 42.6 mg/dL, P < 0.05) and mixed localization of follicles (mucosa and submucosa) (8 vs 1, P < 0.05) than patients with ≤ 2 follicles. Subjects with mixed localized lymphoid follicles showed a statistically significant lower hemoglobin (11.9 ± 1.4 g/L vs 14 ± 13 g/L, P = 0.03) and serum β-carotene (44 ± 21.9 mg/dL vs 111.2 ± 38.7 mg/dL, P < 0.02) as well as a tendency to prolong thromboplastin time (17.3 ± 7.2 vs 11.5 ± 2.5, P < 0.07) than those with exclusively mucosa-localized follicles.
Villous atrophy (partial or total) was observed in seven cases (38%) and it was associated with weight loss (7 vs 2, P < 0.02), lientery (4 vs 0, P < 0.07) and low globulin level (2.3 ± 0.87 g/L vs 3.2 ± 5.2 g/L, P < 0.06). This group had also lower hemoglobin (12.3 ± 1.3 g/L vs 14.2 ± 14 g/L, P < 0.03) and cholesterol serum level (170 ± 38.1 mg vs 200 ± 17.1 mg, P < 0.03) than those without atrophy (Table 2).
| Abnormality | n (%) |
| Localization of lymphoid follicles | |
| • Mucosa | 13 (69) |
| • Mucosa and submucosa | 5 (31) |
| Inflammatory changes in lamina propria | |
| Chronic | 18 (100) |
| • Lymphocytes-plasma cells | 10 (53) |
| • Plasma cells | 7 (38) |
| • Lymphocytes | 1 (9) |
| Acute and chronic | 2 (15) |
| Villous atrophy | 7 (38) |
| • Partial | 5 (23) |
| • Total | 2 (15) |
| Increased intraepithelial lymphocytes | 2 (15) |
| Crypt hyperplasia | 1 (9) |
NLH was associated with hypogammaglobulinemia in 9 (50%) patients, CVI in 8 cases and selective IgA deficiency in 1 case. In this group the mean level of globulins was 1.6 ± 3.5 g/L which was quite different from that in subjects with normal globulins (3.2 ± 4.5 g/L, P < 0.001).
Giardia lamblia infection was found in only one case. E. coli, Streptococcus sp, and Klebsiella sp were respectively isolated from duodenal fluid in three (17%) (106 bacterial colonies per milliliter). These patients showed a markedly prolonged prothrombin time (18.5 ± 7.4 s vs 11.4 ± 1.9 s, P < 0.014). Anti-endomysium antibodies were tested in 16 subjects and two (12.5%) were positive. Both had villous atrophy thus the diagnosis of celiac sprue was established.
Lymphoma was found in three (17%) cases: one with nodular sclerosing-Hodgkin´s disease on the mediastinum, one with jejunum non-Hodgkin’s lymphoma (diffuse large B-cell lymphoma) and one with gastric lymphoma. Patients with lymphoma were older (58 ± 10 years old) and only one of them presented diarrhea as a predominant symptom. No AIDS-associated NLH was found in this series (Table 3).
| Disorder | n |
| Hypogammaglobulinemia | 9 |
| Lymphoma | 3 |
| Intestinal bacterial overgrowth | 3 |
| Celiac sprue | 2 |
| Giardiasis | 1 |
| Graves disease | 1 |
| MNGIE | 1 |
| Chronic pancreatitis | 1 |
| Pancreas divisum | 1 |
Nodular lymphoid hyperplasia (NLH) is a well-characterized condition frequently associated with increased risk of gastrointestinal tumors, mainly gastrointestinal lymphoma. As a pathological entity it seems to be rare. It has been described in immunocompromised and immunocompetent subjects, normal children and adults and patients with AIDS[1-5].
This series represent the whole experience of the National Institute of Health in Mexico, which is a tertiary referral center for gastrointestinal diseases. In previous works Canto et al[6] and Castañeda et al[7] have described some cases with NLH seen at the same institution. In these two papers 12 patients are included in a period of 18 years contrasting with the 18 cases found by us in only a 5-year period. This difference could be explained by the selection criteria used. While Canto et al[6] selected the cases based on the radiological and histological findings. We included only the cases who met the strict histological criteria from a large database of subjects with duodenal biopsy. On the other hand, upper GI endoscopy with biopsy and duodenal fluid aspirate was performed in each patient with chronic diarrhea. NLH was found in 18 cases (4%). This low frequency can be explained by our selection criteria. Again we only included subjects with a duodenal biopsy with or without small bowel radiology. It was reported that NLH is most frequently located in jejunum-ileum[1-3].
The pathogenesis of NLH is unknown but is likely the result of an accumulation of plasma-cell precursors due to a maturational defect in the development of B-lymphocytes in order to compensate for functionally inadequate intestinal lymphoid tissue[8]. The role of functionally inadequate intestinal lymphoid tissue is suggested by the common occurrence of hypogammaglobulinemia and recurrent infections (particularly by Giardia lamblia)[9]. Nine patients had disgammaglobulinemia, but we were able to demonstrate Giardia lamblia infection only in one. It should be noted that we only used stool analysis for ova and parasites and microscopic duodenal fluid examination. It is possible that some cases could be missed because we did not perform fecal ELISA for giardia-specific antigen, which is the most sensitive and specific method for giardia detection[10]. Another plausible explanation is the high frequency of auto-medication used in our patients. Mexican subjects with chronic diarrhea are empirically treated with antibiotics including metronidazole.
Histological diagnosis of NLH can demonstrate the hyperplasic lymphoid follicles with mitotically active germinal centers at mucosa and/or submucosa. The number of follicles is not a diagnostic criterion[1,2]. We found a mean of 2.5 hyperplasic lymphoid follicles in a single 4× magnification field. Patients with three or more localized hyperplasic follicles in mucosa or submucosa showed markers of poor intestinal absorption such as low serum hemoglobin and β-carotene and a marked prolonged prothrombin time, suggesting that fat malabsorption may in turn lead to deficiencies of fat-soluble vitamins. These patients also had chronic diarrhea and a marked weight loss.
The association between NLH and other malignant and benign diseases has been clearly described[9,12-15]. In our series, seven cases had CVI and one had selective IgA deficiency. CVI is a heterogeneous form of immunodeficiency associated with decreased serum immunoglobulin levels, recurrent sinopulmonary infections, gastrointestinal disorders, and increased frequency of malignancies[12,13]. The association between NLH, hypogammaglobulinemia and Giardia lamblia infection is known as Herman’s syndrome[8]. We only found one case with disgammaglobulinemia, NLH and Giardia lamblia infection. The only real difference with Canto’s work was the low detection of giardiasis in our series, a feature which is needed to establish the diagnosis of Herman’s syndrome.
The risk of malignancy has been well recognized in subjects with NLH[14]. Three of our cases had lymphoma. Lymphoma has been reported in patients with and without immunodeficiency[15,16]. The link between the extra intestinal lymphoma and NLH is less clear. We found one patient with Hodgkin’s disease localized on the mediastinum. Jonsson et al[4] have reported a case of extra intestinal lymphoma associated with NLH. Hyperplasic tissue completely disappeared after chemotherapy with remission of lymphoma and then reappeared at relapse.
To the best of our knowledge, no relationship between NLH and celiac disease has been reported. In our series, intestinal atrophy, increased intraepithelial lymphocytes and anti-endomysium antibodies were found in two patients. One of them was asymptomatic on gluten-free diet, and complete resolution of anatomical changes was found in duodenal-specimens taken two months later. In the other case, no clinical response was found six weeks after implementation of a gluten-free diet. This patient lost her follow-up and we do not know her current histological status. Moderate to severe intestinal atrophy and increased intraepithelial lymphocytes have been reported in patients with CVI known as “pseudo-celiac” pattern[17]. In contrast to the typical changes seen in patients with celiac disease, CVI cases showed mild inflammatory infiltrate at the lamina propria with normal enterocyte maturation. We identified partial villous atrophy in five patients with negative anti-endomysium antibodies. Perhaps these 5 patients correspond to the pseudo-celiac pattern previously described.
S- Editor Guo SY L- Editor Wang XL E- Editor Bi L
| 1. | Rambaud JC, De Saint-Louvent P, Marti R, Galian A, Mason DY, Wassef M, Licht H, Valleur P, Bernier JJ. Diffuse follicular lymphoid hyperplasia of the small intestine without primary immunoglobulin deficiency. Am J Med. 1982;73:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Ranchod M, Lewin KJ, Dorfman RF. Lymphoid hyperplasia of the gastrointestinal tract. A study of 26 cases and review of the literature. Am J Surg Pathol. 1978;2:383-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 80] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 8-1997. A 65-year-old man with recurrent abdominal pain for five years. N Engl J Med. 1997;336:786-793. [PubMed] |
| 4. | Jonsson OT, Birgisson S, Reykdal S. Resolution of nodular lymphoid hyperplasia of the gastrointestinal tract following chemotherapy for extraintestinal lymphoma. Dig Dis Sci. 2002;47:2463-2465. [PubMed] [DOI] [Full Text] |
| 5. | Levendoglu H, Rosen Y. Nodular lymphoid hyperplasia of gut in HIV infection. Am J Gastroenterol. 1992;87:1200-1202. [PubMed] |
| 6. | Canto J, Arista J, Hernández J. [Nodular lymphoid hyperplasia of the intestine. Clinico-pathologic characteristics in 11 cases]. Rev Invest Clin. 1990;42:198-203. [PubMed] |
| 7. | Castañeda-Romero B, Díaz-Caldelas L, Galván-Guerra E, Sixtos S, Arista J, Uscanga L. [Intestinal lymphoid nodular hyperplasia in a patient with acquired dysgammaglobulinemia, chronic diarrhea, and bacterial overgrowth syndrome]. Rev Gastroenterol Mex. 1993;58:225-228. [PubMed] |
| 8. | Hermans PE, Diaz-Buxo JA, Stobo JD. Idiopathic late-onset immunoglobulin deficiency. Clinical observations in 50 patients. Am J Med. 1976;61:221-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 224] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | de Weerth A, Gocht A, Seewald S, Brand B, van Lunzen J, Seitz U, Thonke F, Fritscher-Ravens A, Soehendra N. Duodenal nodular lymphoid hyperplasia caused by giardiasis infection in a patient who is immunodeficient. Gastrointest Endosc. 2002;55:605-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Vesy CJ, Peterson WL. Review article: the management of Giardiasis. Aliment Pharmacol Ther. 1999;13:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Al Samman M, Zuckerman MJ, Mohandas A, Ting S, Hoffpauir JT. Intestinal nodular lymphoid hyperplasia in a patient with chronic diarrhea and recurrent sinopulmonary infections. Am J Gastroenterol. 2000;95:2147-2149. [PubMed] |
| 12. | Cooper MD, Schoerder HW. Primary immune deficiency diseases. In: Kasper DL, ed. Harrison’s Principles of Internal Medicine, 16th ed. McGraw-Hill 2005; 1939-1947. |
| 13. | Lai Ping So A, Mayer L. Gastrointestinal manifestations of primary immunodeficiency disorders. Semin Gastrointest Dis. 1997;8:22-32. [PubMed] |
| 14. | Ryan JC. Premalignant conditions of the small intestine. Semin Gastrointest Dis. 1996;7:88-93. [PubMed] |
| 15. | Matuchansky C, Touchard G, Lemaire M, Babin P, Demeocq F, Fonck Y, Meyer M, Preud'Homme JL. Malignant lymphoma of the small bowel associated with diffuse nodular lymphoid hyperplasia. N Engl J Med. 1985;313:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Castellano G, Moreno D, Galvao O, Ballestín C, Colina F, Mollejo M, Morillas JD, Solís Herruzo JA. Malignant lymphoma of jejunum with common variable hypogammaglobulinemia and diffuse nodular hyperplasia of the small intestine. A case study and literature review. J Clin Gastroenterol. 1992;15:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Washington K, Stenzel TT, Buckley RH, Gottfried MR. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol. 1996;20:1240-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 184] [Article Influence: 6.3] [Reference Citation Analysis (0)] |