Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1941
Revised: July 20, 2005
Accepted: July 28, 2005
Published online: March 28, 2006
AIM: To evaluate the prevalence of Giardia lamblia (G. lamblia) infection in patients with irritable bowel syndrome (IBS) and dyspepsia and to establish which is the most accurate test to diagnose the infection in this setting.
METHODS: One hundred and thirty-seven patients who consecutively attended the Outpatient Gastroenterology Clinic for the first time between January 2002 and December 2003 due to symptoms of IBS and/or dyspepsia were recruited. All patients underwent clinical evaluation, first-step haematology and chemistry tests, serologic assays for celiac disease, lactose-H2 breath test, abdominal ultrasonography, and esophagogastroduodenoscopy. Helicobacter pylori status was evaluated. In patients with symptoms of IBS older than 45 years, colonoscopy was also performed. In all patients, duodenal biopsies and stool samples were examined for trophozoites and cysts of G. lamblia by several methods.
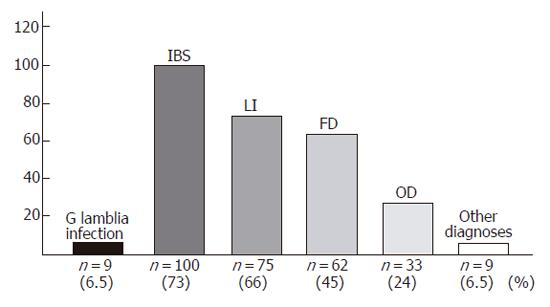
RESULTS: G. lamblia was identified in 9 patients. The following diagnoses were also made: IBS (100/137, 73%), functional dyspepsia (62/137, 45%), organic dyspepsia (33/137, 24%), and lactose intolerance (75/137, 55%). A significant association was found between giardiasis and H pylori infection (χ2 = 6.632, OR = 12.4, CI = 1.5-68.1). There were no symptoms that reliably allowed the recognition of giardiasis. Direct search of the parasite in duodenal biopsy and stool sample examinations gave concordant results in all cases while histological examination of duodenal biopsies displayed a low sensitivity (e.g., 22.2%).
CONCLUSION: In this consecutive series, diagnosis of G. lamblia infection accounted for 6.5% of patients with IBS and dyspepsia. Duodenal biopsies for diagnosis of giardiasis may be unnecessary if stool sample examination is performed.
- Citation: Grazioli B, Matera G, Laratta C, Schipani G, Guarnieri G, Spiniello E, Imeneo M, Amorosi A, Focà A, Luzza F. Giardia lamblia infection in patients with irritable bowel syndrome and dyspepsia: A prospective study. World J Gastroenterol 2006; 12(12): 1941-1944
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1941.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1941
Giardia lamblia is a cosmopolitan parasite with worldwide distribution and the most common protozoan isolated from gastrointestinal tract[1]. The prevalence of infection varies from 2%-7% in industrialized countries to 40% in tropical/subtropical regions with poor sanitation and hygienic conditions[2-5].
Giardiasis typically occurs following the ingestion of water or foods contaminated with fecal material containing cysts, and the infective dose may be as low as 10 cysts. The clinical aspects of giardiasis are largely nonspecific. The infection can be asymptomatic or patients may present with extraintestinal symptoms, such as fever, maculopapular rashes, pulmonary infiltrates, lymphadenopathy, polyarthritis and urticaria. However, the most common symptoms are diarrhea, abdominal pain, bloating, flatulence and weight loss resulting from malabsorption[6]. Since these symptoms may overlap those of patients with other gastrointestinal disorders[7-9], it is not clear when the infection should be suspected. Classically, diagnosis of giardiasis is performed by microscopic examination of stool samples and/or duodenal biopsies and further methods include immunocromathography and immunofluorescence on stool samples. There are few studies comparing the diagnostic proficiency of the methods using either stool or duodenal biopsy samples.
The aim of this study was to evaluate the prevalence of G. lamblia infection in patients with irritable bowel syndrome (IBS) and dyspepsia and to establish the most accurate test for its diagnosis in this setting.
Four hundred and thirty-five consecutive patients attending our hospital for the first time between January 2002 and December 2003 on two days of the week (Monday and Wednesday) due to symptoms of IBS who satisfied Rome II criteria[10] and/or dyspepsia were considered for the study. Patients with alarm features (such as weight loss, dysphagia, vomiting, bleeding), familiarity for gastrointestinal neoplasia and inflammatory bowel disease were excluded as well as patients who took nonsteroidal anti-inflammatory drugs and proton pump inhibitors in the last 15 days and antibiotics in the last 30 days. Patients with symptoms lasting more than one year were also excluded. Finally, 137 patients (48 M, 89 F; median age 39 years, range 19-77 years) were enrolled.
All patients underwent routine blood investigations, including erythrocyte sedimentation rate, C-reactive protein, thyroid hormones, antigliadin, antiendomysial and antitransglutaminase antibodies. Furthermore, urinalysis, microscopic examination of stools, esophagogastroduod-enoscopy with duodenal biopsy, evaluation of Helicobacter pylori (H pylori) infection, abdominal ultrasonography, and lactose-H2 breath test were performed. Patients with symptoms of IBS older than 45 years (n = 46) underwent also colonoscopy.
The presence of the parasite was evaluated by three different approaches: direct search of the parasite in the duodenal biopsy samples with or without use of fixation and dyes, histological examinations of other aliquots of the above bioptic samples, and parasitological evaluations of stool samples.
Search of the parasite in duodenal biopsy samples: Duodenal biopsies were obtained during the upper gastrointestinal endoscopy, immediately submitted to microbiology laboratory where they were put into a sterile plastic container filled with 10 mL of sterile saline. The samples were processed within 2 h of arrival in the laboratory. Wet mount preparations were obtained by cutting the original biopsy into 0.5 mm pieces on a glass slide, with the help of a razor blade and a plastic pipette tip. Then such pieces were suspended in 2 drops of sterile saline and a cover glass was put on them. Microscopic examination for motile organisms was carried out using low and high dry power (× 200 and × 400). Permanent stains were prepared as reported above. However the biopsy pieces were air dried, fixed and the giemsa (Giemsa Plus, Trend Scientific) or trichrome staining (Trichrome Stain, Scientific Device Laboratory Inc., Des Plaines, IL, USA) were carried out following the manufacturer’s instructions. Also the vital staining with acridine orange was used on unfixed specimens examined by confocal microscopy to help identify the parasite and address both the mobility and some details of its cytostructure.
Histology of bioptic samples: Duodenal biopsies obtained during the upper gastrointestinal endoscopy were also submitted to the Pathology laboratory, where the specimens where paraffin-included, stained with hematoxylin-eosin and examined for Giardia trophozoites as well as for inflammatory infiltrate particularly of plasma cellular type.
Stool parasitology: Liquid or semi liquid fecal specimens were immediately submitted to the microbiology laboratory, a wet mount with saline and Lugol’s iodine (Dobell reactive) was prepared and microscopic examination for motile organisms and Giardia cysts was carried out using low and high dry power (× 200 and × 400). Moreover, both liquid and solid fecal samples were processed using the formalin/ether enrichment method followed by microscopy for Giardia cysts. Coproantigens were also evaluated in the stool specimens by immunochromatographic technique (Xpect, Remel Europe, Dartford Kent, UK), an EIA method (Prospect, Remel Europe, Dartford Kent, UK), and commercial direct fluorescent antibody assay (Merifluor, Meridian Bioscience Inc., Cincinnati, USA). Laboratory tests for Giardia coproantigens were carried out and the results were evaluated following the manufacturer’s instructions.
The microbiologist (GM) and pathologist (AA) were blinded to the clinical and laboratory data.
Patients were classified as H pylori positive when the urease quick test (Yamanouchi Pharma, Milan, Italy) was positive. Patients underwent C-13 urea breath test (Cortex, Milan, Italy) when the urease quick test was negative and were considered free of the infection when the two tests were both negative for H pylori.
The relation between presence of giardiasis and H pylori infection was evaluated with χ2test and odds ratio (OR). Data were given together with 95% confidence interval (CI).
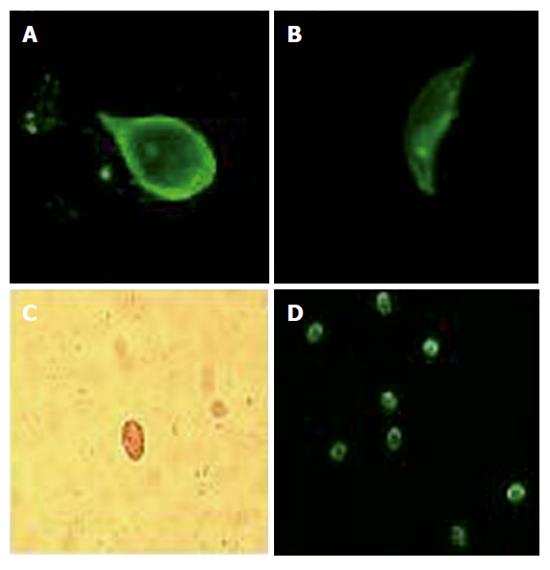
G. lamblia infection was found in 9 of the 137 patients (6.5%). According to the clinical and laboratory evaluations, the following diagnoses were also made: IBS, lactose intolerance, functional and organic dyspepsia, colon diverticulosis, celiac disease, inflammatory bowel disease, gastric cancer, colon cancer, and collagenous colitis (Figure 1). All patients with giardiasis (7 M, 2 F; median age: 38 years, range 26-61 years) had nonspecific (mostly mild) symptoms largely overlapping those complained by patients without giardiasis (Table 1). Indeed, according to the clinical and laboratory findings, these patients were considered affected also by other functional and organic disorders (Table 1). In patients with giardiasis, blood examination was normal with only one patient showing a moderate eosinophilia (1.190/μL; normal range: 0-500/μL). Direct search of the parasite in duodenal biopsy (Figures 2A, 2B) and stool sample (microscopic) examination (Figures 2C, 2D) were concordantly positive (9 of 137 patients) and negative (128 of 137 patients) for G. lamblia, while histological evaluation of duodenal biopsy was positive in only two cases (2 of 137 patients) and negative in the remaining (135 of 137 patients) (Table 2). Using as reference the former two tests, the sensitivity and specificity of the histological examination of duodenal biopsy for the diagnosis of giardiasis were 22.2% and 100%, respectively.
| Patient | Age (yr) | Sex | Symptoms | IBS | LI | FD | OD |
| 1 | 61 | M | Abdominal pain, constipation/diarrhea | + | - | + | - |
| 2 | 38 | F | Abdominal pain, diarrhea, pirosis | + | + | - | + |
| 3 | 33 | M | Abdominal pain, diarrhea | + | - | - | - |
| 4 | 26 | F | Abdominal pain and bloating, constipation | + | + | - | - |
| 5 | 48 | M | Abdominal pain, diarrhea | + | + | - | - |
| 6 | 47 | M | Abdominal pain, diarrhea, pirosis | + | - | + | - |
| 7 | 37 | M | Abdominal pain and bloating, diarrhea | + | - | - | + |
| 8 | 26 | M | Abdominal pain and bloating, diarrhea | + | + | + | - |
| 9 | 39 | M | Abdominal pain, diarrhea | + | - | - | - |
| Patient | Age (yr) | Sex | Direct search induodenal biopsy | Stool sampleexamination | Histology ofduodenal biopsy |
| 1 | 61 | M | + | + | - |
| 2 | 38 | F | + | + | - |
| 3 | 33 | M | + | + | + |
| 4 | 26 | F | + | + | - |
| 5 | 48 | M | + | + | - |
| 6 | 47 | M | + | + | - |
| 7 | 37 | M | + | + | + |
| 8 | 26 | M | + | + | - |
| 9 | 39 | M | + | + | - |
Fifty-eight patients (42%) were infected with H pylori (18 M, 40 F; median age 40 years, range 20-65 years). A significant association was found between giardiasis and H pylori infection. Eight of 58 patients (14%) with H pylori infection had giardiasis while 1 of 79 (1%) without H pylori infection had giardiasis (χ2 = 6.632, OR = 12.4, CI = 1.5-68.1).
This study aimed at investigating the impact of G. lamblia infection on patients who complained of symptoms referring to IBS and dyspepsia. In order to prevent information and observational bias, we properly excluded patients who have had a previous gastroenterological consultation, those with symptoms lasting more than one year and those who had been recently taking drugs that might interfere with complaints and laboratory investigations. Furthermore, to minimize the presence of serious illness, patients complaining of alarm symptoms were excluded. In the consecutive series of 137 patients with symptoms of IBS and/or dyspepsia, the prevalence of giardiasis was 6.5% (9/137). Possibly due to the selection of a symptomatic study population, this figure is slightly higher than the average in the general population in a Western country[2-5]. Nevertheless, no comparisons can be made with the incidence of giardiasis in the asymptomatic population coming from our geographical area.
Furthermore, an important finding of this prospective study is that no symptoms could reliably discriminate patients with giardiasis from those without the parasite. Giardiasis may be present in patients with other gastrointestinal disorders as observed in this study, it seems that symptoms of one disease may overcome/overlap those of the other. Nevertheless, the prevalence of giardiasis in patients suffering from IBS and/or dyspepsia can be considered as the cause of 1BS and/or dyspepsia.
Another important finding of this study involves the diagnostic procedures to be taken into account when dealing with G. lamblia infection. The data showed that histological examination of duodenal biopsies for G. lamblia was unsuitable due to an unacceptable rate of false negative results (e.g., 22.2% sensitivity). At the same time, it has been shown that stool examination is as accurate as the direct search of the parasite in the duodenal samples. Consequently, when a skilled microbiologist and appropriate techniques are available, duodenal biopsy seems unnecessary in accurate identification of G. lamblia, thus invasive and expensive tools such as upper gastrointestinal endoscopy could be avoided.
This study also showed that giardiasis was significantly associated with H pylori infection, possibly reflecting that the two infections share a number of risk factors[11]. This association may have several clinical implications in regard to the transmission mode[12,13], the possibility of a synergy in metronidazole resistance[14], and the experimental evidence of a common pathogenesis scenario, leading to gastrointestinal metaplasia[15].
In conclusion, the prevalence of giardiasis in patients suffering from symptoms of IBS and dyspepsia is non-negligible, and duodenal biopsies for the parasite may be unnecessary if stool examination is performed.
S- Editor Guo SY L- Editor Wang XL E- Editor Bi L
| 1. | Adam RD. The biology of Giardia spp. Microbiol Rev. 1991;55:706-732. [PubMed] |
| 2. | Meyer EA. The epidemiology of Giardiasis. Parasitol Today. 1985;1:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Flanagan PA. Giardia--diagnosis, clinical course and epidemiology. A review. Epidemiol Infect. 1992;109:1-22. [PubMed] |
| 4. | Scotti S, Pettoello Mantoani M, Polito G, Carlomagno F, Coppola A, di Martino L. [Giardia duodenalis infections in pediatrics: our series]. Infez Med. 1996;4:35-40. [PubMed] |
| 5. | Odoi A, Martin SW, Michel P, Holt J, Middleton D, Wilson J. Determinants of the geographical distribution of endemic giardiasis in Ontario, Canada: a spatial modelling approach. Epidemiol Infect. 2004;132:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Garcia LS. Diagnostic medical parasitology. American Society for microbiology. Washington D. 2001;36-49. |
| 7. | D'Anchino M, Orlando D, De Feudis L. Giardia lamblia infections become clinically evident by eliciting symptoms of irritable bowel syndrome. J Infect. 2002;45:169-172. [PubMed] |
| 8. | Carr MF Jr, Ma J, Green PH. Giardia lamblia in patients undergoing endoscopy: lack of evidence for a role in nonulcer dyspepsia. Gastroenterology. 1988;95:972-974. [PubMed] |
| 9. | Rana SV, Bhasin DK, Vinayak VK. Lactose hydrogen breath test in Giardia lamblia-positive patients. Dig Dis Sci. 2005;50:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43-II47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 830] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 11. | Moreira ED Jr, Nassri VB, Santos RS, Matos JF, de Carvalho WA, Silvani CS, Santana e Sant'ana C. Association of Helicobacter pylori infection and giardiasis: results from a study of surrogate markers for fecal exposure among children. World J Gastroenterol. 2005;11:2759-2763. [PubMed] |
| 12. | Ashbolt NJ. Risk analysis of drinking water microbial contamination versus disinfection by-products (DBPs). Toxicology. 2004;198:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Leclerc H, Schwartzbrod L, Dei-Cas E. Microbial agents associated with waterborne diseases. Crit Rev Microbiol. 2002;28:371-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Land KM, Johnson PJ. Molecular basis of metronidazole resistance in pathogenic bacteria and protozoa. Drug Resist Updat. 1999;2:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Patterson MM, Schrenzel MD, Feng Y, Fox JG. Gastritis and intestinal metaplasia in Syrian hamsters infected with Helicobacter aurati and two other microaerobes. Vet Pathol. 2000;37:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |