Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1937
Revised: August 22, 2005
Accepted: August 31, 2005
Published online: March 28, 2006
AIM: To investigate the expression and activity of NAD(P)H quinone oxidoreductase 1 (NQO1) in human liver specimens obtained from patients with liver damage due to acetaminophen (APAP) overdose or primary biliary cirrhosis (PBC).
METHODS: NQO1 activity was determined in cytosol from normal, APAP and PBC liver specimens. Western blot and immunohistochemical staining were used to determine patterns of NQO1 expression using a specific antibody against NQO1.
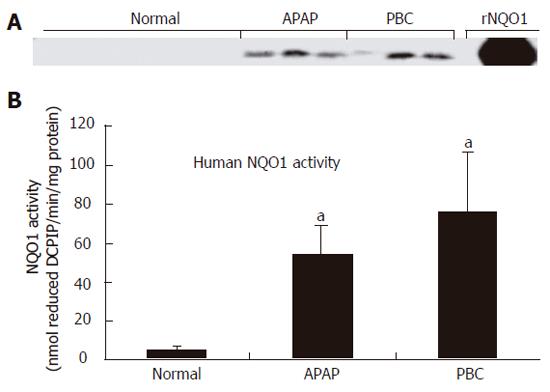
RESULTS: NQO1 protein was very low in normal human livers. In both APAP and PBC livers, there was strong induction of NQO1 protein levels on Western blot. Correspondingly, significant up-regulation of enzyme activity (16- and 22-fold, P < 0.05) was also observed in APAP and PBC livers, respectively. Immunohistochemical analysis highlighted injury-specific patterns of NQO1 staining in both APAP and PBC livers.
CONCLUSION: These data demonstrate that NQO1 protein and activity are markedly induced in human livers during both APAP overdose and PBC. Up-regulation of this cytoprotective enzyme may represent an adaptive stress response to limit further disease progression by detoxifying reactive species.
- Citation: Aleksunes LM, Goedken M, Manautou JE. Up-regulation of NAD(P)H quinone oxidoreductase 1 during human liver injury. World J Gastroenterol 2006; 12(12): 1937-1940
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1937.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1937
Although NAD(P)H quinone oxidoreductase 1 (NQO1) has been historically associated with generation of hydroquinones from reactive quinones, additional substrates including nitrosoamine, nitro and azo chemical moieties have been identified. NQO1 is also capable of scavenging superoxide anions generated during oxidative stress and regenerating reduced forms of protective endogenous antioxidant compounds.
There is very low expression of NQO1 mRNA and protein in normal human livers, with slightly greater mRNA levels observed in biliary epithelial cells[1,2]. NAD(P)H quinone oxidoreductase 2 (NQO2) mRNA is greater in hepatocytes compared to NQO1 mRNA and is in turn thought to play a more critical role in maintaining low levels of quinones in hepatocytes[2]. Consequently, it has been suggested that human NQO1 does not play a major role in hepatic xenobiotic metabolism under normal conditions[3].
Instead, NQO1 may be more important during periods of hepatic oxidative stress and damage. NQO1 mRNA protein and activity are markedly increased in mouse liver following bile duct ligation, a model of obstructive cholestasis (José Manautou, unpublished observations). Similar elevations in rodent NQO1 mRNA also occur after exposure to centrilobular hepatotoxicants such as acetaminophen (APAP), carbon tetrachloride and bromobenzene[4,5]. Although different regions of the liver lobule are injured in cholestasis and drug-induced hepatocellular necrosis, oxidative stress contributes to the pathogenesis of both diseases. We hypothesize that up-regulation of NQO1 may represent a common response to liver injury with an oxidative stress component.
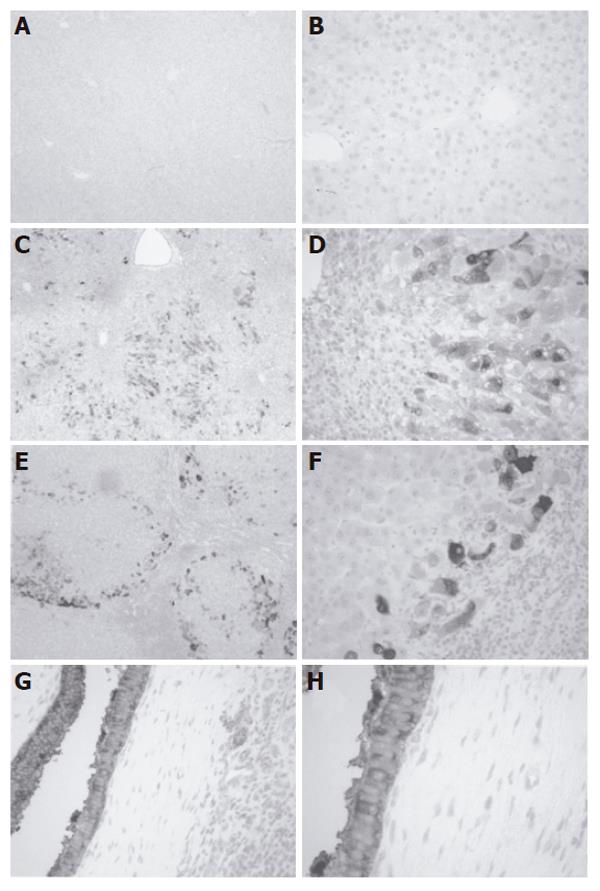
The present study was undertaken to determine if NQO1 expression and activity are similarly altered in two types of human liver disease, injury by APAP overdose and primary biliary cirrhosis (PBC). The results showed that protein levels and activity of human hepatic NQO1 were elevated during APAP overdose and PBC. Immunohistochemical analysis highlighted injury-specific patterns of NQO1 staining. APAP livers showed loss of centrilobular cells and NQO1 staining in midzonal and periportal hepatocytes. Conversely, PBC livers exhibited prominent staining of hyperplastic biliary epithelium and hepatocytes at the periphery of cirrhotic nodules.
2,6-dichlorophenol-indophenol (DCPIP), dicumarol, sucrose, Tris-hydrochloride, nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) were all obtained from Sigma Chemical Co. (St. Louis, MO).
Archival samples of frozen and formalin-fixed, paraffin-embedded adult livers (normal, primary biliary cirrhosis and APAP overdose) were obtained from the Liver Tissue Procurement and Distribution System at the University of Minnesota which was funded by NIH Contract #N01-DK-9-2310. Diagnosis was first established by the University of Minnesota and confirmed by histological examination of tissue sections at the University of Connecticut in a blinded fashion. Patient characteristics are provided in Table 1.
| Patient | Gender | Age | Diagnosis |
| 1 | F | 54 | Normal liver |
| 22 | F | adult | Normal liver |
| 3 | M | 21 | Normal liver |
| 4 | M | 20 | Normal liver |
| 5 | M | 18 | Normal liver |
| 61 | F | 33 | Fulminant hepatic-failure (APAP-induced) |
| 71 | F | 35 | Fulminant hepatic-failure (APAP-induced) |
| 81 | M | 15 | Fulminant hepatic-failure (APAP-induced) |
| 91 | F | 55 | Primary biliary cirrhosis |
| 101 | F | 62 | Primary biliary cirrhosis |
| 111 | F | 63 | Primary biliary cirrhosis |
Livers were homogenized in sucrose-Tris buffer (0.25 mol/L sucrose, 10 mmol/L Tris–HCl, pH 7.4) and centrifuged at 105 000 r/min for 60 min at 4°C. The resulting supernatant contained the cytosolic fraction. Protein concentrations were determined by the method of Lowry using Bio-Rad protein assay reagents (Bio-Rad Laboratories, Hercules, CA). Anti-NQO1 monoclonal antibodies (clones A180 and B771) were kindly provided by David Ross (University of Colorado, Health Science Center, Denver, CO). Cytosolic proteins (40 µg protein/lane) were electrophoretically resolved using polyacrylamide gels (12% resolving, 4% stacking) and transblotted overnight at 4ºC onto PVDF-Plus membrane (Micron Separations, Westboro, MA). Immunostaining was performed as previously described[1]. NQO1 protein-antibody complexes were detected using an ECL chemiluminescent kit (Amersham Life Science, Arlington Heights, IL) and exposed to Fuji medical X-ray film (Fisher Scientific, Springfield, NJ). Equal protein loading was confirmed by Coomassie blue staining of blots (data not shown). Antibody specificity was confirmed using 2.5 μg recombinant human NQO1 (rNQO1)(Sigma Chemical Co. St. Louis, MO) as a positive control.
NQO1 activity was calculated by measuring the colorimetric oxidation of NADPH to NADP+ using DCPIP as substrate. The disappearance of NADPH was measured at 600 nm for over 1 min as described by Ernster with modifications by Benson[6,7]. NQO1 activity was measured in 1-mL reactions (27°C) containing liver cytosol, 200 µmol/L NADPH, 40 µmol/L DCPIP and Tris-HCl buffer (25 mmol/L Tris-HCl, pH 7.4, 0.7 mg/mL bovine serum albumin). Parallel reactions were performed in the presence of 20 µmol/L dicumarol. The rate of dicumarol-sensitive NQO1 activity was determined as the difference between the uninhibited and dicumarol-inhibited rates and was normalized to total cytosolic protein as previously described[6].
Immunohistochemistry was performed on tissue sections cut from archival paraffin blocks as previously described[1]. Negative control staining was performed by incubating the sections without primary antibody. The sections were photographed on an Olympus BX50 microscope equipped with a QImaging MicroPublisher 3.3 RTV camera. Images were acquired with QCapture Pro software.
NQO1 activity was presented as mean nmol reduced DCPIP/min/mg protein ± SE (n = 3-5). Differences in NQO1 activity between normal livers and either APAP or PBC livers were determined using Student’s t test. P < 0.05 compared to normal livers was considered statistically significant.
NQO1 expression and activity in normal human liver were very low as previously described[1]. Immunoblot analysis demonstrated marked induction of NQO1 protein in liver cytosol isolated from APAP and PBC specimens (Figure 1A). This increase in protein corresponded to a significant up-regulation of NQO1 activity from 3 ± 2 nmol DCPIP/min/mg protein in normal livers to 45 ± 16 nmol DCPIP/min/mg protein (P < 0.05) and 62 ± 32 nmol DCPIP/min/mg protein (P < 0.05) in APAP and PBC livers, respectively (Figure 1B).
Immunohistochemical staining demonstrated minimal NQO1 expression in hepatocytes and biliary epithelium of normal liver tissue (Figures 2A and 2B). Histology of APAP livers showed centrilobular necrosis and cell loss with lymphocytic accumulations and minimal biliary hyperplasia (Figures 2C and 2D). NQO1 staining was cytosolic and concentrated in midzonal and portal hepatocytes surrounding regions of centrilobular cell loss. Conversely, sections from PBC livers exhibited necrosis, fibrosis and nodular regeneration with lymphocytic-histiocytic infiltration and mild bile stasis (Figures 2E and 2F). Hepatocytes on the periphery of nodules were stained positive for cytosolic NQO1 protein. Positive staining was also observed in hyperplastic biliary epithelium in PBC livers (Figures 2G and 2H). No signal was detected in negative control sections (data not shown).
This is the first report documenting strong induction of NQO1 protein and activity in livers from individuals with two very different types of liver injury; exposure to toxic amounts of APAP or chronic PBC. Drug-induced liver injury is the most common cause of acute liver failure, with APAP exposure accounting for more than 40% of cases[8]. Ingestion of supratherapeutic amounts of APAP results in degeneration and necrosis of centrilobular hepatocytes. Conversely, PBC is a progressive disease primarily attributed to autoimmunity. Chronic inflammation and destruction of bile ducts during PBC cause biliary hyperplasia, marked fibrosis, and cirrhosis in later stages. The specificity of NQO1 up-regulation during liver damage is unknown. Increased NQO1 expression in APAP and PBC livers is similar to that in non-viral human hepatocellular carcinoma[9]. Conversely, livers from patients with type B or C hepatitis-induced hepatocellular carcinoma, focal nodular hyperplasia or cholangiocarcinoma do not demonstrate altered NQO1 levels[2,10]. Together, these and previously reported data suggest differential regulation of NQO1 in human liver disease of varying etiologies.
Transcription factor NFE-2-related factor 2 (Nrf2) is the key regulatory pathway for NQO1 expression. Oxidative stress causes translocation of Nrf2 to the nucleus and binding to antioxidant response elements in the promoter region of numerous detoxification genes, including NQO1[11]. Subsequent gene activation leads to production of NQO1 and other proteins involved in cytoprotection. With the use of Nrf2-null mice, NQO1 expression and induction have been shown to be Nrf2-dependent[12]. Activation of the Nrf2 signaling pathway during different types of human liver disease may be responsible for NQO1 up-regulation in drug-induced damage, cirrhosis and carcinoma.
As an Nrf2 target gene, NQO1 plays a role in cellular antioxidant defense beyond general drug metabolism, which includes dismutation of superoxide[13]. Therefore, NQO1 may be a scavenger of superoxide anions that are generated during both APAP toxicity and PBC[14]. Similarly, NQO1 also limits oxidative stress and lipid peroxidation by converting endogenous antioxidants (such as vitamin E) back to their more active, reduced forms[13,15].
Loss of NQO1 function heightens susceptibility of rodents and humans to a number of diseases as demonstrated in transgenic mice lacking NQO1 and humans with a naturally-occurring NQO1 polymorphism. NQO1-null mice exhibit increased susceptibility to menadione-induced hemolytic anemia while patients possessing a C → T substitution at residue 609 of NQO1 have lower levels of NQO1 protein and exhibit an increased risk of benzene toxicity, urothelial tumors, acute myelogenous leukemia, and numerous additional cancers[13,16,17].
Since the pathogenesis of APAP hepatotoxicity is mediated by an electrophilic quinone, NQO1 might possess the ability to convert the APAP metabolite back to parent compound[18]. Limited data suggest that inhibition of NQO1 by dicumarol potentiates APAP toxicity in mice[19]. Conversely, administration of Nrf2 activating compounds, such as oltipraz, protects hamsters against APAP hepatic damage by inducing detoxification pathways including NQO1[20,21].
The relative constitutive level of hepatic NQO1 among rodents and humans may also explain in part the different susceptibility to APAP hepatotoxicity across species. NQO1 activity is markedly higher in rat liver compared to mouse and human livers. Along the same line, rats exhibit a greater resistance to APAP-induced liver damage compared to mice and humans. Although additional variations in APAP metabolism between rodents and humans are known, differences in basal NQO1 activity among these species may also be a determinant of susceptibility to APAP.
In conclusion, NQO1 is upregulated in APAP–induced and cholestatic human hepatic injury, suggesting that this Nrf2 gene may limite the progression of these liver diseases.
The authors thank David Ross and David Siegel for providing the monoclonal NQO1 antibody and procedure for NQO1 immunohistochemistry and Jonathan Maher for technical assistance. Lauren Aleksunes is a Predoctoral Fellow Howard Hughes Medical Institute.
S- Editor Guo SY L- Editor Wang XL E- Editor Bai SH
| 1. | Siegel D, Ross D. Immunodetection of NAD(P)H: quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med. 2000;29:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 215] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Strassburg A, Strassburg CP, Manns MP, Tukey RH. Differential gene expression of NAD(P)H: quinone oxidoreductase and NRH: quinone oxidoreductase in human hepatocellular and biliary tissue. Mol Pharmacol. 2002;61:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H: quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact. 2000;129:77-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 468] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci. 2005;83:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Heijne WH, Slitt AL, van Bladeren PJ, Groten JP, Klaassen CD, Stierum RH, van Ommen B. Bromobenzene-induced hepatotoxicity at the transcriptome level. Toxicol Sci. 2004;79:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Benson AM, Hunkeler MJ, Talalay P. Increase of NAD(P)H: quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980;77:5216-5220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 504] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 7. | Ernster L. DT-Diaphorase. Methods Enzymol. 1967;10:309-317. [RCA] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 442] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Cresteil T, Jaiswal AK. High levels of expression of the NAD(P)H: quinone oxidoreductase (NQO1) gene in tumor cells compared to normal cells of the same origin. Biochem Pharmacol. 1991;42:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Iizuka N, Oka M, Yamada-Okabe H, Hamada K, Nakayama H, Mori N, Tamesa T, Okada T, Takemoto N, Matoba K. Molecular signature in three types of hepatocellular carcinoma with different viral origin by oligonucleotide microarray. Int J Oncol. 2004;24:565-574. [PubMed] |
| 11. | Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H: quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 386] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 12. | Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410-3415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 892] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 13. | Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab Rev. 2004;36:639-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H: quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 361] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D. The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol. 1997;52:300-305. [PubMed] |
| 16. | Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, Gonzalez FJ, Jaiswal AK. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem. 1998;273:7382-7389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 200] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Siegel D, Anwar A, Winski SL, Kepa JK, Zolman KL, Ross D. Rapid polyubiquitination and proteasomal degradation of a mutant form of NAD(P)H: quinone oxidoreductase 1. Mol Pharmacol. 2001;59:263-268. [PubMed] |
| 18. | Powis G, See KL, Santone KS, Melder DC, Hodnett EM. Quinoneimines as substrates for quinone reductase (NAD(P)H: (quinone-acceptor)oxidoreductase) and the effect of dicumarol on their cytotoxicity. Biochem Pharmacol. 1987;36:2473-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lee SM, Cho TS, Kim DJ, Cha YN. Protective effect of ethanol against acetaminophen-induced hepatotoxicity in mice: role of NADH: quinone reductase. Biochem Pharmacol. 1999;58:1547-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Davies MH, Schnell RC. Oltipraz-induced amelioration of acetaminophen hepatotoxicity in hamsters. II. Competitive shunt in metabolism via glucuronidation. Toxicol Appl Pharmacol. 1991;109:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Davies MH, Schamber GJ, Schnell RC. Oltipraz-induced amelioration of acetaminophen hepatotoxicity in hamsters. I. Lack of dependence on glutathione. Toxicol Appl Pharmacol. 1991;109:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |