Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1933
Revised: July 2, 2005
Accepted: July 15, 2005
Published online: March 28, 2006
AIM: To assess the causes of ileocecal mass in patients with amebic liver abscess.
METHODS: Patients with amebic liver abscess and ileocecal mass were carefully examined and investigated by contrast-enhanced CT scan followed by colonoscopy and histological examination of biopsy materials from lesions during colonoscopy.
RESULTS: Ileocecal masses were found in seventeen patients with amebic liver abscess. The cause of the mass was ameboma in 14 patients, cecal tuberculosis in 2 patients and adenocarcinoma of the cecum in 1 patient. Colonic ulcers were noted in five of the six (83%) patients with active diarrhea at presentation. The ileocecal mass in all these patients was ameboma. Ulcers were seen in only one of the 11 (9%) patients without diarrhea. The difference was statistically significant from the group with diarrhea (P < 0.005).
CONCLUSION: Ileocecal mass is not an uncommon finding in patients with amebic liver abscess. Although, the ileocecal mass is due to ameboma formation in most cases, it should not be assumed that this is the case in all patients. Colonoscopy and histological examination of the target biopsies are mandatory to avoid missing a more sinister lesion.
- Citation: Misra SP, Misra V, Dwivedi M. Ileocecal masses in patients with amebic liver abscess: Etiology and management. World J Gastroenterol 2006; 12(12): 1933-1936
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1933
Intestinal amebiasis occurs throughout the world[1]. It is estimated that out of all people infected with E. histolytica, 40 million develop colitis or extra-intestinal abscesses and of these 40 000 die annually[1]. Extra-intestinal amebiasis is also not uncommon and the liver is the most common extra-intestinal organ to be involved[1-3]. A post-mortem study reported that the liver is involved in 37% of cases[4]. Involvement of the colon is also common in patients with amebic liver abscess (ALA)[5,6]. A recent study showed that colonic ulcers are observed in 55% of patients with ALA. These ulcers are more common in patients with active diarrhea or history of diarrhea in the recent past[6]. Some of the patients with ALA also have a palpable ileocecal mass on abdominal examination. The cause of the ileocecal mass in these patients still remains unclear.
Formation of an ameboma has been noted to be an uncommon complication of amebiasis[7-18]. It has been estimated that ameboma occurs in only about 1.5% of all cases[8]. Although there have been recent reports dealing with the problem of ameboma[14,17], in regions where ALA is not very common, ameboma has been confused with cancer of the colon[9,13,14,18]. However, in regions where ALA is common, the reverse may happen and if diagnostic facilities are not widely available as in the case of several developing countries, cancer of the colon or tuberculosis may be confused with ameboma, especially in patients with concurrent ALA and its clinical signs and symptoms of high fever, local pain and tenderness. We therefore report our findings in patients with amebic liver abscess(es) and associated cecal mass. We believe that this is the largest collection of patients with ameboma.
Consecutive patients with ALA having a palpable ileocecal mass were studied. The diagnosis of ALA was made on the basis of the clinical features of fever, right upper abdominal pain, tender hepatomegaly with a typical ultrasonic finding of a single or multiple hypoechoeic lesion(s) in the liver and antiamebic antibody titer >160 in serum (immunofluorescent assay).
A detailed clinical history was obtained from all patients and recorded. Patients were asked specifically about loose stools (three or more in a day for more than 48 h), with or without blood during the last eight weeks. Whole stool samples were observed and three fresh samples were examined for the presence of trophozoites and cysts of E. histolytica.
Contrast-enhanced CT scan was performed for all patients and apart from the abscess in the liver, the ileocecal area was carefully studied.
Colonoscopy was carried out in all but one patient within two days of instituting anti-amebic treatment after stool samples were obtained for microscopic examination. In one patient colonoscopy could be performed on the fifth day of instituting anti-amebic therapy.
Colonic preparation was done for all patients using a polyethylene glycol-electrolyte-based solution (Peglec, Tablets India Ltd, Chennai). The procedure was performed under conscious sedation with intravenous diazepam (5-10 mg) and pentazocine (25-50 mg). The findings observed during colonoscopy were recorded. The site, size, number and presence of inflammation around the ulcers were recorded if ulcers were noted. Multiple biopsies were obtained from the ulcer margins for histopathological examination. When the cecum was reached, the area was carefully inspected and multiple biopsies were obtained from any lesion that was encountered.
Patients who were too sick, those who did not give consent, those with ruptured ALA, those requiring urgent needle or catheter drainage and those with severe comorbid illnesses, were excluded from the study. Patients who received anti-amebic treatment during the preceding eight weeks were also excluded.
The protocol for the study was cleared by the Ethical Committee of the Hospital and a detailed informed consent was obtained from all participating patients.
A total of 17 patients with ALA and ileocecal mass were studied. There were 16 males and one female. The mean (SD) age of these patients was 37.9 (9.9) years (range 24 to 62 years). Two patients presented with active lower gastrointestinal bleeding.
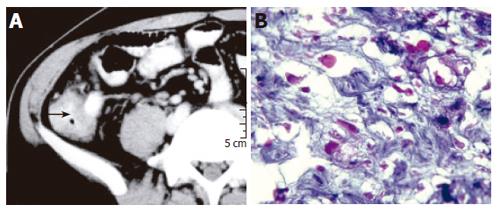
Contrast-enhanced CT scan demonstrated rounded hypodense lesions in the liver due to ALA (Figure 1). In all the patients, thickening of the cecal wall was noted (Figure 2A). Lymphnodal enlargement was not evident and a definite diagnosis could be made on the basis of the CT findings in none of the patients.
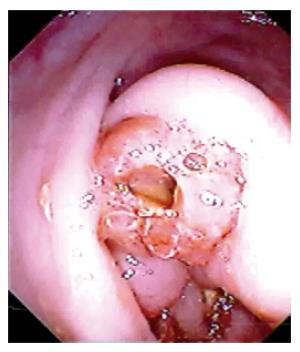
Diarrhea was present in six patients. The other 11 patients did not complain of diarrhea. Ulcers were noted in the colon of five of the six (83%) patients with active diarrhea. The ileocecal mass in all these patients was due to ameboma formation (Figure 2B). Ulcers were seen in only one of the 11 (9%) patients without diarrhea. The difference between the two groups was statistically significant (P < 0.005, Fisher’s exact test).
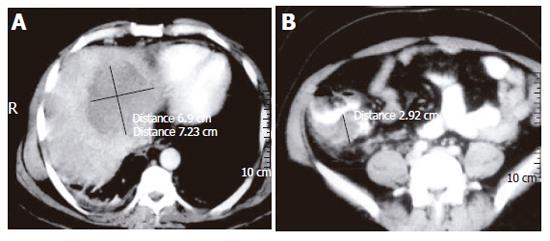
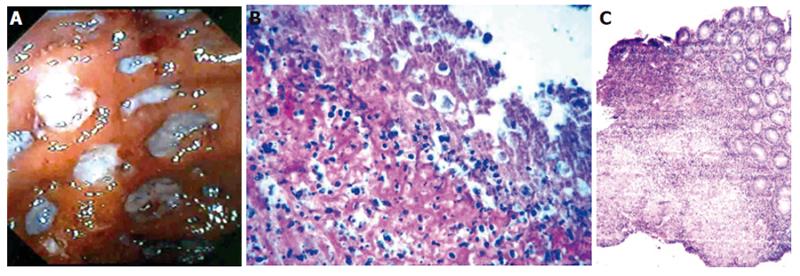
At colonoscopy, sixteen patients showed mass lesions in the cecum. The patient who underwent colonoscopy on the fifth day of starting anti-amebic therapy showed evidence of amebic liver abscess (Figure 3A) and thickening of the cecal wall on contrast-enhanced CT scan (Figure 3B), but colonoscopy showed multiple round and oval, deep ulcers of varying sizes confined to the cecum and the adjoining ascending colon (Figure 4A). Colonic biopsies obtained from the ulcers showed trophozoites of E. histolytica (Figure 4B).
The colonoscopic appearance in all patients, except for one who underwent delayed examination, showed mass lesions in the cecum and the colonoscopic appearance was similar in all of them. The final diagnosis of ileocecal mass was made after histological examination of the tissue obtained at colonoscopy. The final diagnosis was ameboma in 14 patients, ileocecal tuberculosis in two patients (Figure 4C) and adenocarcinoma of the cecum in one patient.
The patient with cancer of the colon was a 32-year-old man who was admitted due to massive lower GI bleeding. At the time of hospitalization, the pulse rate was 160/min. The systolic blood pressure was 70 mm Hg. The hemoglobin was 5 g/dL. The patient was in shock and managed with intravenous fluids and blood transfusion. After haemodynamic stability was achieved, emergency colonoscopy was performed, which showed that the bleeding originated from the ascending colon. The bleeding stopped spontaneously on the next day. An ultrasound examination of the abdomen showed a large hypoechoeic lesion in the left lobe of the liver, consistent with the diagnosis of amebic liver abscess. CT scan showed similar findings. In addition to the lesion in the liver, there was thickening of the wall of the cecum. No other abnormality was detected. Aspiration of the liver abscess yielded an anchovy sauce-like material. Cytological examination of the aspirate revealed acute inflammatory cells. No amebic trophozoites or malignant cells were seen. Indirect hemagglutination test for presence of antibody against E. histolytica was positive in titres of 1:160.
Repeat colonoscopy following bowel preparation showed irregular, nodular friable growth in the cecum (Figure 5). Multiple biopsies were obtained from the growth. Histological examination of the biopsies revealed adenocarcinoma. The patient was advised to undergo right hemi-colectomy but did not consent to be operated. The amebic liver abscess responded to needle aspiration and metronidazole.
A 62- year old lady who was diagnosed to have ALA five months ago received anti-amebic therapy at another hospital and was reported to have been cured of the disease. She however, visited our hospital with diarrhea, high fever and a mass in the right lower quadrant. Ultrasonography and CT scan of the abdomen showed features of ALA. CT scan also showed thickening of the cecal wall. Colonoscopy showed a friable nodular mass in the cecum. A provisional diagnosis of cancer of the cecum was made. Histological examination of biopsies obtained from the cecal mass during colonoscopy, however, showed trophozoites of E. histolytica and the mass was finally diagnosed as an ameboma.
All the patients received metronidazole. Nine patients required needle aspiration of ALA. All patients were cured of ALA. The ileocecal masses due to ameboma formation disappeared after treatment with metronidazole in all the patients. Repeat colonoscopy did not reveal any abnormality in 12 patients and mild hyperemia in the remaining two patients with ameboma. The two patients with ileocecal tuberculosis received anti-tuberculous treatment and were well during follow-up. The cecal masses were no longer palpable after follow-up of 6 and 7 months respectively.
Although ALA is rare in developed countries, it is not an infrequent disease in developing countries. However, the occurrence of ileocecal mass due to ALA is rare, except for a case report[11]. If an ileocecal mass is detected in a patient with ALA, an ameboma should be diagnosed.
It has been estimated that out of all cases with amebiasis, ameboma formation occurs in only about 1.5% of patients[8]. However, it is important to note that in regions where ALA is not very common, ameboma has been confused with cancer of the colon[9,13,14,18]. More importantly, in regions where ALA is common, the reverse may happen and cancer of the colon or tuberculosis may be confused with ameboma, especially in patients with concurrent ALA and its clinical signs and symptoms of high fever, local pain and tenderness. This was evident in one patient in this series who had carcinoma of the cecum but was clinically thought to have an ameboma due to the young age and concurrent occurrence of ALA. Two other patients in this series were clinically thought to have ameboma of the cecum on the basis of the colonoscopic findings, but showed tuberculosis of the cecum on histological examination of colonoscopic biopsies. In an elderly patient, a clinical diagnosis of cecal cancer was considered and it was only on histological examination of the biopsies that the diagnosis of ameboma could be made.
It is generally considered that ameboma formation occurs due to untreated or partially treated amebic colitis[1,7]. However, in the present series, only one patient had a history of anti-amebic treatment five months ago for ALA. None of the other patients received any anti-amebic treatment in the recent past. Moreover, all of them had evidence of ALA at the time when they were found to have an ileocecal mass, suggesting that ameboma formation can occur even in the acute or subacute phase.
CT findings of cecal ameboma with pericolonic lessions have been reported recently[17]. The ileocecal valve shows a large abscess within the mesentry causing compression of the adjacent terminal ileum and cecum. Even then a differential diagnosis of lymphoma, neoplasm, enterocolitis and inflammatory bowel disease are considered. It was reported that due to concentric thickening of the cecal wall and mass like appearance, a clinical diagnosis of ameboma could only be established after histopathological examination of colonoscopic biopsies[14]. It is therefore evident that all patients with ileocecal masses, even with concurrent ALA, should undergo colonoscopy and biopsies should be obtained from any suspicious lesions in order not to miss tuberculosis or cancer.
Although in the present series, we encountered only amebomas, cancer of the cecum and tuberculosis as the cause for the ileocecal mass, lymphomas as well as enterocolitis, acute appendicitis, Crohn’s disease, abscess and fungal infections should also be considered as a probable diagnosis.
The simultaneous occurrence of ALA and colonic tuberculosis in this part of the world is not surprising because both diseases are commonly encountered in this region[6,19-21]. This situation may hold true for most developing countries and the two diseases may also occur simultaneously even in developed countries, especially in immigrants, people living in confined areas and patients with immunodeficiency virus infection. The ileocecal mass in patients with cecal tuberculosis is due to proliferative lesions in the cecum resulting in a tumor-like presentation[19]. The cause of the lesions could not be therefore diagnosed on the basis of CT or colonoscopic findings.
As noted in an earlier study[6], the frequency of colonic ulcers is higher in patients with active diarrhea at presentation as opposed to those who had no diarrhea at the time of admission. What was interesting to note is that, in all patients with active diarrhea, the ileocecal mass is due to ameboma formation. Whether active diarrhea in such patients signifies ameboma can only be ascertained if a larger number of patients with similar presentation are studied.
S- Editor Guo SY L- Editor Wang XL E- Editor Bai SH
| 1. | Li E, Stanley SL Jr. Protozoa. Amebiasis. Gastroenterol Clin North Am. 1996;25:471-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Adams EB, MacLeod IN. Invasive amebiasis. II. Amebic liver abscess and its complications. Medicine (Baltimore). 1977;56:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Katzenstein D, Rickerson V, Braude A. New concepts of amebic liver abscess derived from hepatic imaging, serodiagnosis, and hepatic enzymes in 67 consecutive cases in San Diego. Medicine (Baltimore). 1982;61:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | DeBakey Me, Ochsner A. Hepatic amebiasis; a 20 year experience and analysis of 263 cases. Surg Gynecol Obstet. 1951;92:209-231. [PubMed] |
| 5. | Sachdev GK, Dhol P. Colonic involvement in patients with amebic liver abscess: endoscopic findings. Gastrointest Endosc. 1997;46:37-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Misra SP, Misra V, Dwivedi M, Singh PA, Barthwal R. Factors influencing colonic involvement in patients with amebic liver abscess. Gastrointest Endosc. 2004;59:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Marcus VA, Ward BJ, Jutras P. Intestinal amebiasis: a diagnosis not to be missed. Pathol Res Pract. 2001;197:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Cardoso JM, Kimura K, Stoopen M, Cervantes LF, Flizondo L, Churchill R, Moncada R. Radiology of invasive amebiasis of the colon. AJR Am J Roentgenol. 1977;128:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Stuiver PC, Visser LG. [Ameboma of the large intestine and rectum]. Ned Tijdschr Geneeskd. 1993;137:2328-2331. [PubMed] |
| 10. | Recio PM. Ameboma of the colon. Philipp J Surg Surg Spec. 1965;20:61-75. [PubMed] |
| 11. | Sharma D, Patel LK, Vaidya VV. Amoeboma of ascending colon with multiple amoebic liver abscesses. J Assoc Physicians India. 2001;49:579-580. [PubMed] |
| 12. | Majeed SK, Ghazanfar A, Ashraf J. Caecal amoeboma simulating malignant neoplasia, ileocaecal tuberculosis and Crohn's disease. J Coll Physicians Surg Pak. 2003;13:116-117. [PubMed] |
| 13. | Balikian JP, Garabedian MM. Ameboma of the transverse colon simulating carcinoma. Report of two cases with their roentgenologic manifestations. J Med Liban. 1965;18:259-263. [PubMed] |
| 14. | Simşek H, Elsürer R, Sökmensüer C, Balaban HY, Tatar G. Ameboma mimicking carcinoma of the cecum: case report. Gastrointest Endosc. 2004;59:453-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Moschopoulos C, Van Gossum A, Serruys E, Rickaert F, Adler M. [Endoscopic diagnosis of a cecal ameboma successfully treated with antibiotic therapy]. Acta Gastroenterol Belg. 1987;50:685-688. [PubMed] |
| 16. | Luterman L, Alsumait AR, Daly DS, Goresky CA. Colonoscopic features of cecal amebomas. Gastrointest Endosc. 1985;31:204-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Stockinger ZT. Colonic ameboma: its appearance on CT: report of a case. Dis Colon Rectum. 2004;47:527-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Guzmán Valdivia Gómez G, Chavelas Lluck M, Medina González E. [Unsuspected tumor of the colon]. Rev Gastroenterol Mex. 1996;61:362-365. [PubMed] |
| 19. | Misra SP, Misra V, Dwivedi M, Gupta SC. Colonic tuberculosis: clinical features, endoscopic appearance and management. J Gastroenterol Hepatol. 1999;14:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Misra SP, Dwivedi M, Misra V, Gupta M, Kunwar BK. Endoscopic biopsies from normal-appearing terminal ileum and cecum in patients with suspected colonic tuberculosis. Endoscopy. 2004;36:612-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Misra SP, Misra V, Dwivedi M, Arora JS, Kunwar BK. Tuberculous colonic strictures: impact of dilation on diagnosis. Endoscopy. 2004;36:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |