Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1912
Revised: November 2, 2005
Accepted: November 26, 2005
Published online: March 28, 2006
AIM: To study the effect of WeiJia on chronic liver injury using carbon tetrachloride (CCl4) induced liver injury animal model.
METHODS: Wistar rats weighing 180-220g were randomly divided into three groups: normal control group (Group A), CCl4 induced liver injury control group (Group B) and CCl4 induction with WeiJia treatment group (Group C). Each group consisted of 14 rats. Liver damage and fibrosis was induced by subcutaneous injection with 40% CCl4 in olive oil at 3 mL/kg body weight twice a week for eight weeks for Groups B and C rats whereas olive oil was used for Group A rats. Starting from the third week, Group C rats also received daily intraperitoneal injection of WeiJia at a dose of 1.25 μg/kg body weight. Animals were sacrificed at the fifth week (4 male, 3 female), and eighth week (4 male, 3 female) respectively. Degree of fibrosis were measured and serological markers for liver fibrosis and function including hyaluronic acid (HA), type IV collagen (CIV), γ-glutamyl transferase (γ-GT), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined. Alpha smooth muscle actin (α-SMA) and proliferating cell nuclear antigen (PCNA) immunohistochemistry were also performed.
RESULTS: CCl4 induction led to the damage of liver and development of fibrosis in Group B and Group C rats when compared to Group A rats. The treatment of WeiJia in Group C rats could reduce the fibrosis condition significantly compared to Group B rats. The effect could be observed after three weeks of treatment and was more obvious after eight weeks of treatment. Serum HA, CIV, ALT, AST and γ-GT levels after eight weeks of treatment for Group C rats were 58±22 µg/L (P<0.01), 57±21 µg/L (P<0.01), 47±10 U/L (P<0.01), 139±13 U/L (P<0.05) and 52±21 U/L (P>0.05) respectively, similar to normal control group (Group A), but significantly different from CCl4 induced liver injury control group (Group B). An increase in PCNA and decrease in α-SMA expression level was also observed.
CONCLUSION: WeiJia could improve liver function and reduce liver fibrosis which might be through the inhibition of stellate cell activity.
- Citation: Cheung PY, Zhang Q, Zhang YO, Bai GR, Lin MCM, Chan B, Fong CC, Shi L, Shi YF, Chun J, Kung HF, Yang M. Effect of WeiJia on carbon tetrachloride induced chronic liver injury. World J Gastroenterol 2006; 12(12): 1912-1917
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1912.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1912
Hepatic fibrosis is one of the processes that occur when the liver is damaged through viral activity, toxins, autoimmune diseases, metabolic disorder or genetic defects. It is a result of chronic liver injury that ultimately leads to cirrhosis and its complications of portal hypertension, liver failure and hepatocellular carcinoma. Millions of people die each year worldwide[1-5]. Efficient and well-tolerated antifibrotic drugs are lacking and current treatment of hepatic fibrosis is limited to withdrawal of the noxious agent[6].
Advance in pathophysiology, molecular biology, genetically engineered animals, liver transplantation etc. has increased our understanding of the molecular mechanism in liver fibrogenesis. Hepatic stellate cell (HSC) was identified as the primary cell type to mediate fibrogenesis which is the major source of extracellular matrix deposition[7-9]. Antifibrotic drug development was focused on fibrogenic cells generating the scarring response in the past decade.
WeiJia is a protein peptide mixture extracted from neonate porcine liver. It is a Category-I new drug on the market approved by the State Food and Drug Administration (SFDA) for the treatment of severe hepatitis and shows promising results in clinical studies[10-12]. Studies also showed that WeiJia can act as a therapeutic agent in the treatment of cirrhosis[13,14]. As progressive hepatic fibrosis would lead to cirrhosis, it is likely that WeiJia might also play a role in treatment of hepatic fibrosis. In this study, carbon tetrachloride (CCl4) induced liver injury animal model was used to evaluate the potential of WeiJia as a therapeutic agent for hepatic fibrosis.
Wistar rats, weighing 180-220g were bought from Experimental Animal Centre, Daping Hospital, Third Military Medical University, Chongqing. WeiJia was from LifeTec Enterprise Limited, Hong Kong; analytical grade CCl4, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GT) testing kits and DAB were from Sigma (St. Louis, MO, USA); hyaluronic acid (HA) and type IV collagen (CIV) RIA kits were from Shanghai Naval Medical Research Institute; alpha smooth muscle actin (α-SMA) and proliferating cell nuclear antigen (PCNA) monoclonal antibodies were from Dako Ltd. (Glostrup, Denmark)
Wistar rats were randomly divided into three groups: normal control group (Group A), CCl4 induced liver injury control group (Group B) and CCl4 induction with WeiJia treatment group (Group C). Each group consisted of 14 rats (8 male, 6 female). Liver damage and fibrosis was induced by subcutaneous injection with 40% CCl4 in olive oil at 3 mL/kg body weight twice a week for eight weeks for Group B and C rats whereas olive oil alone was used for Group A rats. Rats were allowed to feed ad libitum. Starting from the third week, Group C rats also received daily intraperitoneal injection of WeiJia at a dose of 1.25 µg/kg body weight. All studies were conducted according to the guidelines described in the NIH Guide for the Care and Use of Laboratory Animals.
Half of the rats (4 male, 3 female) in each group were sacrificed at the fifth week and the other half were sacrificed at the eighth week. Amobarbital sodium (0.5%) was injected intraperitoneally into the rats at 4 mL/kg and blood was collected from the heart after anesthesia. Blood (5 mL) was collected and 1.2 mL serum was obtained after centrifugation and stored at 4 °C before analysis. Liver tissues (1 cm×1 cm×1 cm) from the right liver lobe were dissected and immobilized in 4% paraformaldehyde. Tissue was then embedded in paraffin wax and sectioned (4 µm thick) before analysis.
Liver fibrosis blood tests for HA and CIV were performed using competitive RIA method. Blood serum level of γ-GT, ALT and AST were measured by standard clinical chemical methods using an automatic analyzer type ALCYON 300i (Abbott Laboratories Ltd, USA).
Tissue was sectioned, haematoxylin and eosin (HE) staining, Van Gieson (VG) staining and immunohistochemistry were performed and examined under light microscope. All histological examinations were performed by experienced pathologist without prior knowledge of the animal treatment groups in the study. Images were acquired through Nikon Eclipse E400 (Nikon Corporation, Japan) and analyzed with analySIS 3.0 software.
Degree of fibrosis was measured on HE stained sections. Stage of liver fibrosis was graded with the METAVIR scale, which grades fibrosis on a five-point scale: F0 (no fibrosis), F1 (portal fibrosis without septa), F2 (portal fibrosis with a few septa), F3 (numerous septa without cirrhosis) and F4 (cirrhosis). METAVIR scale is a widely used scale that has excellent inter-observer reliability[15,16].
Ballooning degeneration and steatosis for HE stained sections were graded according to a four point scale where Grade 0: negative, Grade (1): up to 33%, Grade (2): 33%-66% and Grade (3): > 66% cells show ballooning degeneration and steatosis[17].
The collagen content of the sections was determined by VG staining. Five random fields were chosen in each section and the amount of total collagen was detected as the area stained by VG and expressed as percentage relative to the total area.
α-SMA and PCNA immunohistochemistry were also performed. Sections were deparaffinized, rehydrated and incubated in 3% hydrogen peroxide at room temperature for 10 min to block endogenous peroxidase. After rinsing with distilled water, sections were incubated in phosphate buffered saline (PBS, 0.01 mol/L, pH 7.4) for 5 min and epitope retrieval was induced with heat in a microwave oven. Non-specific binding sites were blocked with 10% normal goat serum (NGS) / 10% bovine serum albumin (1∶1 dilution) for 30 min at room temperature followed by incubation with monoclonal mouse anti α-SMA or PCNA as primary antibodies at 1∶100 dilution in PBS containing 10% NGS and 0.3% Tween 20 overnight at 4 °C. Sections were then washed with PBS for 3 times, each 5 min before applying the secondary antibody. Goat anti-mouse antibody conjugated with horseradish peroxidase (HRP) at 1∶200 dilution in PBS containing 10% NGS was applied and the sections were incubated for 30 min at 37 °C. Sections were then washed with PBS for 3 times, each 5 min and stained with DAB for 1 to 5 min. Staining was stopped by washing with tap water. Sections were counterstained with haematoxylin, dried and visualized under light microscope.
Expression of α-SMA was determined according to four categories and each category was assigned a number. Grade (1): no expression (-, 20=1); Grade (2): individual positive cells expressed in diseased area (+, 21=2); Grade (3): a few positive cells gathered together and expressed in the diseased area (++, 22=4) and Grade (4): wide spread of positive cells (+++, 23=8). Result is expressed in numbers according to different categories.
Expression of PCNA was determined using a double blind method. For each section, 5 random fields at high resolution were chosen and positive cells were recorded by two analysts. Result is expressed as the mean positive cells recorded by the two analysts.
Comparison of the degree of liver fibrosis between samples was performed by WILCOXON method. Other data were analyzed by SPSS11.0 software and reported as mean + standard deviation using one-way ANOVA. Student’s t-test was used for comparison between groups. P values of 0.05 or less are considered statistically significant.
WeiJia is an SFDA-approved Category-I new drug for the treatment of severe hepatitis. WeJia showed an overall efficacy of 88.9% in relieving symptoms and improving physical conditions of chronic hepatitis patients in a treatment period of six weeks in previous clinical study. Studies also showed that WeiJia can act as a therapeutic agent in the treatment of cirrhosis[13,14]. As progressive hepatic fibrosis would lead to cirrhosis, it is likely that WeiJia might also play a role in the treatment of hepatic fibrosis. Thus the effect of WeiJia on liver fibrosis was investigated through an animal model in this study.
Rats were induced with CCl4 followed by the treatment with WeiJia. Animals without CCl4 induction or without WeiJia treatment were used as control for comparison. The effect of CCl4 and WeiJia on rat liver fibrosis was determined through histological examination and serological markers test.
Serum levels of biochemical markers were determined to evaluate the severity of fibrosis. Levels of extracellular matrix constituents HA and CIV were measured which wer expected to increase as a result of remodelling and recurrent scarring in liver fibrogenesis. HA has correlation with stage 3 and 4 fibrogenesis. Together with CIV and other markers, differentiation of stage 1 and 2 fibrosis from stage 3 and 4 fibrosis can be obtained[18,19]. HA and CIV serum levels for different treatment groups are shown in Table 1. Significant elevation of serum HA and CIV levels were observed upon CCl4 induction (P < 0.001 vs Group A). Their levels were significantly reduced upon treatment with WeiJia (P < 0.005 vs Group B). Though there was still significant difference between the levels of Group C and Group A rats at the fifth week, there was no apparent difference for CIV level at the eighth week between the two groups, indicating prolonged treatment with WeiJia could alleviate the severity of fibrosis. Decrease of the CIV and HA levels were observed for Group B rats at the 8th wk when compared to their level at the 5th wk indicating some recovery processes took place. However, such recovery processes were not potent enough to revert or alleviate the severity of fibrosis as their enzyme levels were still significantly higher than that of Group A rats.
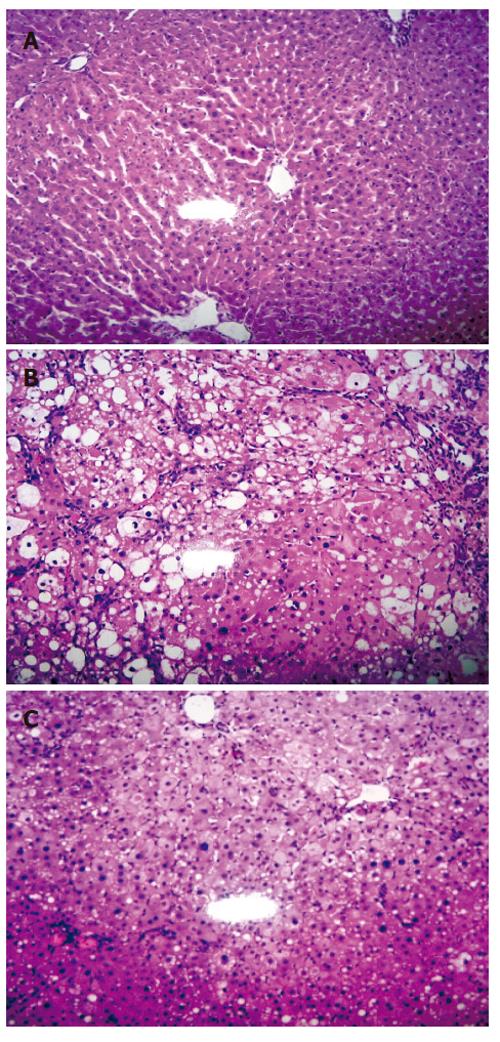
The great elevation of HA and CIV levels upon CCl4 induction indicated the successful generation of liver fibrosis animal model. The result was further confirmed by histopathology study. HE staining for sections of normal control group (Group A) showed structural integrity without necrosis, inflammation or fibrosis development. VG staining for collagen was negative too. However, CCl4 induced liver injury control group (Group B) showed significant fibrosis (P < 0.01) with the loss of structural integrity and formation of nodules that lacked a central vein. Steatosis and ballooning degeneration (P < 0.01) were observed on the fifth week whereas increased collagen fibres and complete fibrous septa were observed on the eighth week. Nevertheless, CCl4 induction with WeiJia treatment group (Group C) showed that WeiJia could significantly alleviate CCl4 induced alterations as seen in Group B rats. No obvious changes for fibrosis were observed on the fifth week (P > 0.05). However, the condition for ballooning degeneration and steatosis were significantly relieved at this stage (P < 0.05). After prolonged treatment, a significant reduction in inflammation, steatosis (P < 0.05), necrosis, fibrosis (P < 0.01) and collagen fibres (P < 0.05) were observed on the eighth week. Representative HE stained images of sample on the eighth week are shown in Figure 1. There was no apparent difference between male and female rats. The degree of fibrosis, ballooning degeneration and steatosis and collagen expression are summarized in Table 2, Table 3 and Table 4.
The effect of CCl4 on liver and the role of WeiJia in treating liver fibrosis were also determined through liver function test. Serum levels of ALT, AST and γ-GT were measured and compared with control groups as shown in Table 5.
Significant increase in serum levels of ALT, AST and γ-GT upon CCl4 induction were observed (P<0.005 Group B vs Group A). A significant decrease in the levels of ALT and AST were observed after WeiJia treatment (P<0.05 Group C vs Group B). However, the decrease was not significant for γ-GT (P > 0.1 Group C vs Group B). This indicates that there was no strong correlation between the degrees of fibrosis and γ-GT as γ-GT is an indicator for primary or metastatic malignancy involving liver[20].
It has been known that ALT and AST are useful serum markers for inflammation and necrosis of the liver. ALT is especially useful in acute and cholestatic diseases whereas AST is more sensitive in chronic and infiltrative lesion of the liver. Though the levels of these enzymes decreased after WeiJia treatment, they were still significantly different when compared to Group A (P < 0.05), except the levels of ALT and γ-GT at the fifth week. Studies have indicated that the ratio rather than the absolute values of the two enzymes provides high specificity in monitoring fibrosis[21-23]. By comparing the ratio of the two enzymes, there was no significant difference between rats treated with WeiJia and that from Group A. The results indicate that WeiJia could alleviate the adverse effect on liver function caused by liver injury.
As WeiJia treatment could alleviate the effects caused by CCl4 induction, it is important to understand how WeiJia mediates its effect. It was suggested that the proliferative rate of regenerating hepatocytes may be an important pathogenetic factor in chronic liver disease[24]. Recent studies have also shown that HSC are the primary cell type in mediating fibrogenesis[7-9]. Thus the expression of PCNA and α-SMA were determined to evaluate the cell proliferation and HSC activation in liver injury respectively. Results are summarized in Table 6.
Normal hepatocytes are generally quiescent and replicate in a limited and regulated manner[25-27]. High proliferative rates were reported in hepatocellular carcinoma, cirrhosis and acute hepatic failure[28-32]. Nevertheless, recent evidences showed that the replicative activity of hepatocytes diminishes in advanced cirrhosis in humans and in chronic liver injury in mouse, reaching a state of replicative senescence[33-35].
Only limited expression of PCNA was found in normal control Group A whereas increased PCNA expression was observed upon CCl4 induction in active hepatocytes nuclei. Significant difference was found at the fifth week (P < 0.05, Group B vs Group A). However, expression of PCNA in Group B at the eighth week was not significantly different from that of Group A. PCNA expression increased further with WeiJia treatment (P < 0.05 Group C vs Groups A and B).
In normal liver, HSCs are nonparenchymal quiescent cells for vitamin A storage. In pathological conditions as in liver fibrosis, HSCs lose retinoids and synthesize a large amount of extracellular matrix components including collagen, proteoglycan and adhesive glycoproteins. Morphology of these cells also changes from the star-shaped stellate cells to that of fibroblasts or myofibroblasts[36]. α-SMA is a good indicator for HSC activation.
Only limited α-SMA expression was observed in normal control group A. Upon CCl4 induction, increased amount of α -SMA expression by activated HSC was observed (P < 0.01 Group B vs Group A). The expression was reduced with WeiJia treatment. However, significant reduction was only observed at the eighth week (P < 0.05 Group C vs Group B). Though WeiJia could reduce α -SMA expression, its level was still significantly different from that of Group A (P < 0.05 Group C vs Group A). The results indicated that WeiJia could mediate the alleviation of CCl4 induced injury through the proliferation of regenerating hepatocytes and the reduction of stellate cell activity.
WeiJia is an effective therapeutic agent for severe hepatitis. However, its action mechanism is not clear. WeiJia was also shown to be effective in cirrhosis treatment. Thus we hypothesize that it may also play a role in fibrosis treatment. In this study, its effect on liver fibrosis was evaluated through CCl4 induced liver injury animal model. This study also provides some information for understanding the mechanism of WeiJia.
It was found that treatment of WeiJia could relieve CCl4 induced liver necrosis, ballooning degeneration, steatosis and inflammation. The effect was significant at an early stage of treatment at the fifth week. An improved liver function was also observed at this stage of treatment. The results suggest that WeiJia protects liver cells from damage induced by CCl4 and the therapeutic effects of WeiJia on severe hepatitis might be related to the protective effect of this medication.
Upon liver injury, the body will attempt to repair the damage through increasing the expression of hepatocyte growth factor (HGF), transforming growth factor beta (TGF-β) and other cytokines to enhance hepatocytes proliferation and initiate tissue repairing process. Replicative activity of hepatocytes diminishes in advanced cirrhosis in humans and in chronic liver injury in mouse [33-35]. PCNA expression in Group B was the response of hepatocyte to liver damage. The level of PCNA expression in Group C was significantly higher than that in Group B suggesting that liver cells of rats in Group C have stronger replicative activity. The increase in PCNA expression in WeiJia treated rats with CCl4 induced liver damage demonstrated that WeiJia has protective effect on liver cells.
Uncontrollable remodelling and regeneration would lead to the development of fibrosis due to excessive deposition of extracellular matrix. The data from this investigation also showed that WeiJia was effective in fibrosis treatment. In addition to inflammation and necrosis, CCl4 induction also resulted in collagen deposition and liver fibrosis as observed through histopathologic examination. Treatment with WeiJia reduced ballooning degeneration, steatosis and accumulation of collagen. The effect was significant at an early stage of treatment at the fifth week. An improved liver function was also observed at this stage of treatment with the increase in PCNA expression. Significant reduction of fibrosis was only observed after a longer period of treatment at the eighth week. Nevertheless, the results indicate that early treatment with WeiJia might prevent the progression of liver injury to fibrosis through increased liver regeneration and reduced liver necrosis.
It is believed that HSC activation is a critical step in hepatic fibrosis. Levels of serological markers (HA, CIV) and α-SMA expression clearly indicated that HSC was activated by CCl4 induced liver injury. WeiJia treatment led to a significant reduction of theses proteins, indicating that the mechanism of WeiJia in reducing hepatic fibrosis may be through the inactivation of HSC.
In conclusion, WeiJia is shown to be an effective therapeutic agent that could alleviate liver fibrosis through the stimulation of liver regeneration and inhibition of HSC activation.
S- Editor Guo SY L- Editor Zhang JZ E- Editor Cao L
| 1. | Lin KW, Kirchner JT. Hepatitis B. Am Fam Physician. 2004;69:75-82. [PubMed] |
| 2. | Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 590] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | McHutchison JG. Understanding hepatitis C. Am J Manag Care. 2004;10:S21-S29. [PubMed] |
| 4. | Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 606] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 5. | McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G497-G502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 6. | Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005;45:605-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000;279:G7-G11. [PubMed] |
| 8. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 9. | Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Yu YY, Si CW, Zeng Z, Wang QH, Luo RD, Zhou YL, Zeng MD, Qiao GY, Yao JL, Chan WL. Clinical research of intravenation of hepatocyte growth promoting factors on hepatitis gravis. J Clin Intern Med. 2002;19:255-257. |
| 11. | Fang H, Xu XJ, Zhou RG. Clinical analysis of hepatocyte growth promoting factors effect on 60 severe chronic hepatitis patients. J Clin Intern Med. 2002;19:474. |
| 12. | Gu WF, Hu XJ, He LM, Wang ZH. Analysis of the therapeutic effect of "weijia" in severe chronic B type viral hepatitis. Linchuang Gandanbing Zazhi. 2003;19:39-40. |
| 13. | Zhong LH, Lu BL Fan Y. The therapeutic effect of hepatocyte growth factor (HGF) in liver cirrhosis after hepatitis. Zhongguo Shenghua Yaowu Zazhi. 2002;23:308-309. |
| 14. | Koenigs JW. Hydrogen peroxide and iron: a microbial cellulolytic system. Biotechnol Bioeng Symp. 1975;151-159. [PubMed] |
| 15. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] |
| 16. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 17. | Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 781] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 18. | Olga OZ, Nikolai DY. Invasive and non-invasive monitoring of hepatitis C virus-induced liver fibrosis: alternatives or complements. Curr Pharm Biotechnol. 2003;4:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 20. | Abbasciano V, Levato F, Zavagli G. Specificity of tumor markers (CEA, GICA, TPA, alpha-FP, FpA, gamma-GT) for the diagnosis of hepatic metastases from large bowel cancers. Med Oncol Tumor Pharmacother. 1989;6:129-132. [PubMed] |
| 21. | Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302-1304. [PubMed] |
| 22. | Imperiale TF, Said AT, Cummings OW, Born LJ. Need for validation of clinical decision aids: use of the AST/ALT ratio in predicting cirrhosis in chronic hepatitis C. Am J Gastroenterol. 2000;95:2328-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Ceppa P, Testa R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Nakamura T, Hayama M, Sakai T, Hotchi M, Tanaka E. Proliferative activity of hepatocytes in chronic viral hepatitis as revealed by immunohistochemistry for proliferating cell nuclear antigen. Hum Pathol. 1993;24:750-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Zajicek G, Oren R, Weinreb M Jr. The streaming liver. Liver. 1985;5:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 180] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Jezequel AM, Paolucci F, Benedetti A, Mancini R, Orlandi F. Enumeration of S-phase cells in normal rat liver by immunohistochemistry using bromodeoxyuridine-antibromodeoxyuridine system. Dig Dis Sci. 1991;36:482-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 517] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 28. | Ballardini G, Groff P, Zoli M, Bianchi G, Giostra F, Francesconi R, Lenzi M, Zauli D, Cassani F, Bianchi F. Increased risk of hepatocellular carcinoma development in patients with cirrhosis and with high hepatocellular proliferation. J Hepatol. 1994;20:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Kawakita N, Seki S, Sakaguchi H, Yanai A, Kuroki T, Mizoguchi Y, Kobayashi K, Monna T. Analysis of proliferating hepatocytes using a monoclonal antibody against proliferating cell nuclear antigen/cyclin in embedded tissues from various liver diseases fixed in formaldehyde. Am J Pathol. 1992;140:513-520. [PubMed] |
| 30. | Seki S, Sakaguchi H, Kawakita N, Yanai A, Kuroki T, Mizoguchi Y, Kobayashi K, Monna T. Detection of proliferating liver cells in various diseases by a monoclonal antibody against DNA polymerase-alpha: with special reference to the relationship between hepatocytes and sinusoidal cells. Hepatology. 1991;14:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Koukoulis G, Rayner A, Tan KC, Williams R, Portmann B. Immunolocalization of regenerating cells after submassive liver necrosis using PCNA staining. J Pathol. 1992;166:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Ojanguren I, Ariza A, Llatjós M, Castellà E, Mate JL, Navas-Palacios JJ. Proliferating cell nuclear antigen expression in normal, regenerative, and neoplastic liver: a fine-needle aspiration cytology and biopsy study. Hum Pathol. 1993;24:905-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Paradis V, Youssef N, Dargère D, Bâ N, Bonvoust F, Deschatrette J, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 221] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Theise ND. Regeneration of hepatocyte 'buds' in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Senoo H. Structure and function of hepatic stellate cells. Med Electron Microsc. 2004;37:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |