Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1889
Revised: October 2, 2005
Accepted: October 9, 2005
Published online: March 28, 2006
AIM: To demonstrate bone marrow stromal cells (BMSCs) can be used as an attractive target for genetic modification in the treatment of malignant diseases.
METHODS: Using a hamster model of biliary cancer, we investigated the therapeutic effects of interleukin-2 (IL-2) gene-modified BMSCs. Syrian golden hamsters were injected via the femoral vein with 5×105 cells of the KIGB-5 biliary cancer cell line (n=20). One week later, the hamsters were injected intraperitoneally with BMSCs containing Ad/hIL-2 and Ad/ΔE1, unmodified BMSCs, or RPMI only (control) and observed for 12 wk (n=5 /each group).
RESULTS: All hamsters treated with BMSCs containing Ad/hIL-2 survived with no evidence of the disease during this period. In contrast, hamsters in the other three groups showed disseminated metastases involving the lungs as early as 4 wk.
CONCLUSION: Ad/IL-2 therapy is effective in the treatment of biliary cancer.
- Citation: Kim MH, Lee SS, Lee SK, Lee SG, Suh CW, Gong GY, Park JS, Kim YH, Kim SH. Interleukin-2 gene-encoded stromal cells inhibit the growth of metastatic cholangiocarcinomas. World J Gastroenterol 2006; 12(12): 1889-1894
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1889
Cholangiocarcinoma arising from bile duct epithelium is the second most common primary liver cancer in the world[1]. In the United States, 2000-3000 new cases of cholangiocarcinoma are diagnosed per year[2]. At present, only surgical excision of all detectable tumors can improve 5-year survival[3-5]. However, most patients are not candidates for surgery and undergo only endoscopic or percutaneous biliary drainage procedures, such as plastic or metal stents. Systemic chemotherapy and radiation therapy are not able to enhance the survival of patients with cholangiocarcinoma[6-11].
Introduction of specific genes into the body by infusion of vehicle cells that have been modified ex vivo offers a potential therapy for a number of diseases. Vehicle cells most commonly used for gene transfer include hematopoietic stem cells, peripheral blood lymphocytes and bone marrow stromal cells (BMSCs). Among these, BMSCs have several advantages including ease of culture and gene transduction, as well as specificity to hematopoietic organs such as bone marrow, spleen, and liver. These qualities make BMSCs ideal for gene transfer in hematologic diseases[12].
BMSCs have been shown to support hematopoiesis and have the potential to differentiate into cells of multiple lineages, including osteogenic, chondrogenic and adipogenic cells[13-15]. Human BMSCs can be easily obtained and grown in conventional ex vivo culture. In addition, BMSCs can be infused safely and have been shown to home to the bone marrow, spleen, lung, liver and kidney. Using a murine lymphoma model, we found that immunocell therapy with ad/hIL-2 encoded stromal cells shows promising therapeutic results[16]. Here we further investigated immunocell therapy for biliary cancer induced by the KIGB-5 cell line in Syrian golden hamsters, and found that Ad/hIL-2 genetically-modified BMSCs could provide adoptive immunocell therapy for biliary cancer.
Female Syrian golden hamsters aged 6-8wk, purchased from Harlan (Indianapolis, Indiana, USA) were housed in the specific pathogen-free unit of the Animal Resource Center at the Asan Institute for Life Sciences and Technology.
The KIGB-5 cell line, a gallbladder carcinoma cell line derived from Syrian golden hamsters, was established and kindly provided by Dr. Tajima (Nagasaki University, Nagasaki, Japan). This cell line was maintained in complete RPMI-1640 (GIBCO BRL, Grand Island, NY, USA) supplemented with 100 mL/L fetal bovine serum (FBS) (GIBCO BRL, Grand Island, NY, USA), 100 U/mL penicillin (Sigma, St. Louis, MO, USA), 0.1 mg/mL streptomycin (Sigma), and 10-5 mol/L β-mercaptoethanol (Sigma)[17]. The 293 cell line, derived from human embryonic kidney, was maintained in high glucose DMEM (GIBCO BRL) and 100 mL/L FBS (GIBCO BRL).
Bone marrow cells were obtained by flushing the femurs and tibias of 6-8 wk old Syrian golden hamsters with RPMI-1640 medium (GIBCO BRL) using 2mL syringes with 23-guage needles. After a single-cell suspension was prepared, the cells were plated at a concentration of 5.0 ×105 /mL in 75 cm2 plastic tissue culture flasks, and cultured in McCoy’s 5A medium (Sigma) supplemented with 125 mL/L FBS, 125 mL/L horse serum (HS), 200mmol/L L-glutamine, 0.05 mg/mL hydrocortisone, 1 ng/mL recombinant human basic fibroblast growth factor (rh bFGF, Invitrogen Corporation, Groningen, Netherlands), and 0.5ng/mL rh IL-1α (Invitrogen) at 37 °C in 50 mL/L CO2 and 950 mL/L air. The cultures were fed weekly by replacing 500 mL/L of the supernatant with fresh culture medium. When the cells became confluent, they were trypsinized and harvested for passage. Gene transduction was performed on cells after 5 passages.
Three replication-deficient adenoviral vectors were used, namely Ad/Lac-Z containing the β-galactosidase gene, Ad/∆E1 and Ad/hIL-2 harboring the human IL-2 gene. Each of these vectors was constructed from human adenovirus serotype 5 by homologous recombination. The expression of the inserted genes was driven by a CMV promoter. The recombinant adenoviruses were propagated into human 293 embryonic kidney cells, and the adenoviral titers were determined by a plaque-forming assay with these cells. Recombinant adenoviruses were diluted to a titer of 5.0 × 108 PFU/mL in viral supernatant and stored at -70 °C.
Stromal cells (SCs) were seeded at a density of 1.0 × 105 /mL in 75 cm2 plastic tissue culture flasks and allowed to grow. The medium was removed, the cells were washed twice with PBS, and the modified adenovirus was added at various multiplicities of infection (MOI). The cells were cultured at 37°C for 24h in a humidified atmosphere of 50 mL/L CO2.
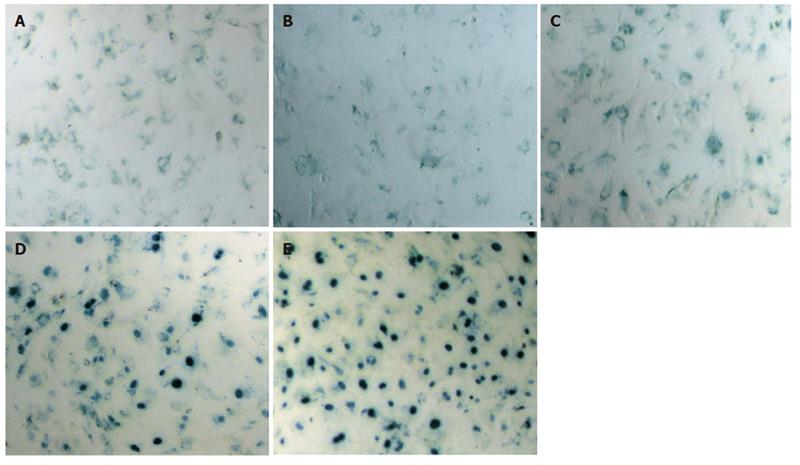
The transduction efficiency of the recombinant adenoviruses was determined by an X-gal assay. BMSCs were transduced with Ad/Lac Z for 24h at 0, 10, 20, 50, 100 MOI. After one round of transfection the transduction efficiency was determined by the percentage of blue-stained cells, after subtraction of the percentage of non-specifically stained, uninfected BMSCs[7]. Stained positive cells were 5-10% at 10 MOI, 10-15% at 20 MOI, 40-50% at 50 MOI, 60-65% at 100 MOI (Figure 1).
From the results of the preliminary experiments we decided to use 50 MOI for further experiments.
BMSCs were seeded in 12-well plates and transfected 24 h later with Ad/Lac Z. The cells were cultured for 24 h at 37 °C in a humidified atmosphere containing 50 mL/L CO2. The media were removed and the cells were washed with PBS. Fixing solution was added to the transfected cells at room temperature for 5 min and removed. The cells were stained with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside(X-gal) overnight at 37°C in a humidified atmosphere containing 50 mL/L CO2.
To test for homing of gene-transduced stromal cells, 6-8 wk old Syrian gold hamsters were infused intraperitoneally (IP) or injected via the femoral vein with transduced stromal cells mixed in 0.1 mL PBS at a dose of 2.5×106 cells per animal. After one week, the hamsters were sacrificed and the bone marrow, spleen, liver, kidneys, and lungs were obtained. Single cells prepared from these organs using collagenase (GIBCO BRL) were cultured for four days and stained with X-gal to test for homing of the transfected cells.
Total RNA was extracted using Trizol (Life Technologies, Invitrogen) following the manufacturer’s instructions. The extracted RNA was resuspended in diethylpyrocarbonate-treated water at a concentration of 0.5 μg/μL. Two ug of each total RNA preparation was reverse-transcribed into cDNA using Moloney murine leukemia virus reverse transcriptase (Boeringer, Manheim, Germany) in 20 μL reaction mixture containing 50 mmol/L DTT, 1 μg oligo (dT) and 0.125 mmol/L dNTPs, as described by the manufacturer. The cDNAs were stored at -20°C or directly used for subsequent amplification. Amplification of human IL-2 mRNA was performed using primers: 5’-TTGCATTGCACTAAGTCTTGC-3’ (forward) and 5’-CAATGGTTGCTGTCTCATCAG-3’ (reverse), whereas amplification of GAPDH mRNA was performed using primers: 5’-ACCACAGTCCATGCCATCAC-3’ (forward) and 5’-TCCACCACCCTGTTGCTGTA-3’ (reverse). PCR was performed in reaction mixtures containing 10 mmol/L Tris-HCl (pH 8.3), 1.5 mmol/L MgCl2, 50 mmol/L KCl, 200 μmol/L of each dNTP, 1 μmol/L of each primer, 0.5 units of Taq polymerase, and 1×concentration of PCR buffer, in a total volume of 50 μL. The amplification protocol consisted of 32 cycles of denaturation at 94°C for 30 s, annealing at 55°C (IL-2) or at 65°C (GAPDH) for 30 s, and extension at 72°C for 40 s (IL-2) or 80 s (GAPDH), using a Perkin-Elmer 9600 thermal cycler. The PCR products were separated on 15 g/L agarose gels stained with ethidium bromide.
BMSCs were seeded at a concentration of 2.5 × 105 cells/mL and transduced with Ad/hIL-2 for 1, 2, 3, or 5 d .The supernatants were collected each day, filtered and tested for the presence of IL-2 by ELISA (Endogen, Woburn, MA, USA).
KIGB-5 cells were cultured for 6-7 passages, washed three times and resuspended in PBS. Each hamster was infused through the femoral vein with 5.0×105 KIGB-5 cells. After seven days, the tumor-bearing hamsters were divided into four groups and injected intraperitoneally with PBS (n = 5), unmodified BMSCs (n = 5), BMSCs+Ad/hIL-2 (n = 5) or BMSCs+Ad/∆E1 (n = 5). Hamsters were sacrificed 4, 8, and 12 wk after tumor inoculation, and the number of metastatic lesions in each was assessed.
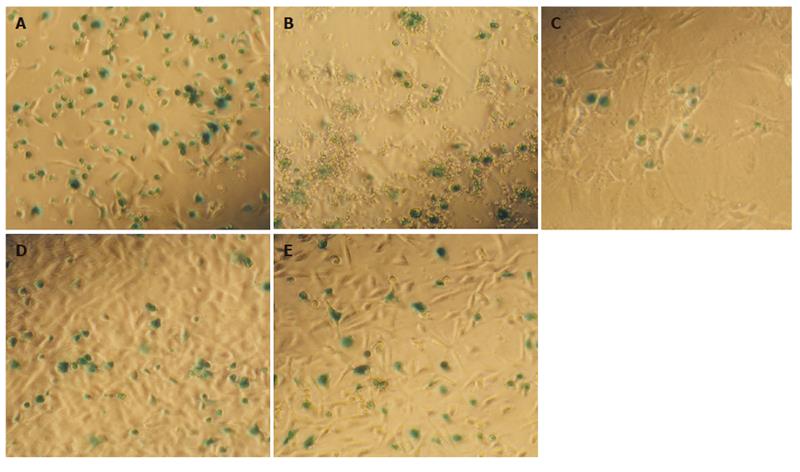
Following transduction of BMSCs with Ad/LacZ at 50 MOI for 24 h, 2.5×106 cells were transplanted into each hamster. After one week, the hamsters were sacrificed and various organs including bone marrow, spleen, kidneys, liver, and lungs, were obtained. Single cell preparations were made from each of these organs and examined by X-gal assay. We found that the BMSCs made in our laboratory homed primarily into the bone marrow, spleen and liver, with fewer in the lungs and kidneys (Figure 2).
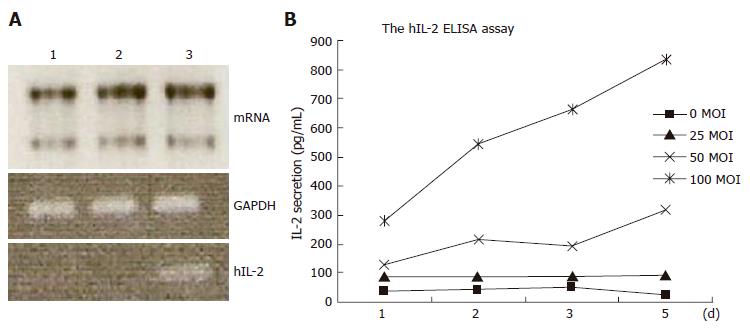
The Ad/hIL-2 construct was designed to express human IL-2 protein in BMSCs. To confirm hIL-2 expression in BMSCs transduced with Ad/hIL-2, we tested these cells by RT-PCR and ELISA. Both showed IL-2 expression in transduced BMSCs (Figure 3).
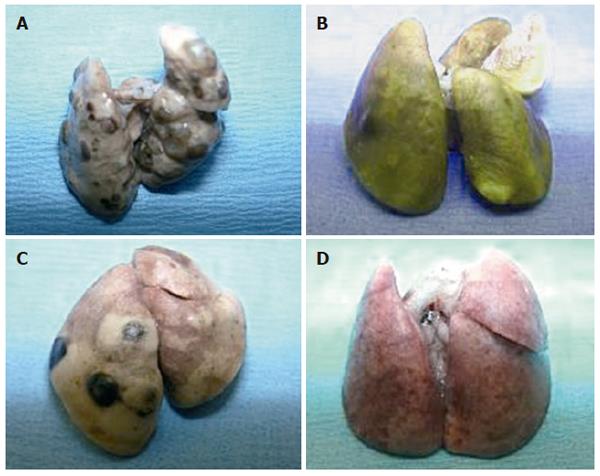
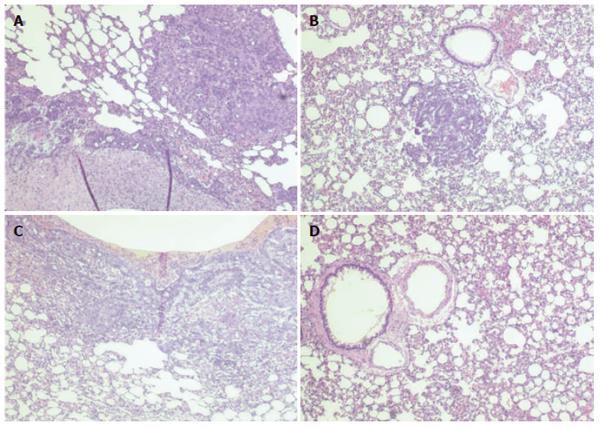
Following injection of KIGB-5 cells into hamsters, we observed gross metastatic lung lesions after 8 wk (Figure 4).
Hamsters subsequently injected with RPMI, unmodified BMSCs, or BMSCs containing Ad/∆E1 had multiple metastatic lung lesions 8 and 12 wk after tumor cell injection, but no metastatic lesions were observed in the liver or other organs. In contrast, no evidence of metastatic tumors was observed in hamsters treated with BMSCs containing Ad/hIL-2. These findings were further confirmed by microscopic examination (Figure 5).
Cholangiocarcinoma is an extremely aggressive tumor most frequently detected at an advanced stage. Fewer than 50% of patients with cholangiocarcinoma have a chance of curative surgery. Patients with advanced disease have a median survival time of less than 6 mo and respond poorly to conventional chemotherapy[18]. Patients who undergo curative surgery have a 5 year-survival rate ranging from 16% to 32% because of early lymph node metastases[19,20].
There have been no clinical trials using adoptive immunotherapy (IL-2 cytokine or dendritic cell and tumor lysates) for biliary cancer. We previously showed that BMSCs can be targeted to bone marrow in mice as well as to other organs, including spleen, liver, kidneys and lungs. Moreover, BMSCs containing Ad/hIL-2, with a low calculated dose of IL-2, are therapeutically effective for malignant lymphoma, because animals treated with this recombinant demonstrate 100% survival with no evidence of the disease, whereas control mice show extensive metastases[16]. These findings encouraged us to investigate whether Ad/hIL-2 would be effective in hamsters bearing biliary cancer induced by the KIGB-5 cell line. IL-2 is a cytokine with multiple biologic effects, including enhancement of the cytotoxic activity of cytotoxic T lymphocytes (CTLC). Previous clinical trials have shown that IL-2 has anti-tumor modulating effects in melanoma, renal cell carcinoma and hematologic malignancies[22]. In each of these models, the IL-2 dose is extremely high and has moderate to severe clinical side effects whereas the response rate ranges from 20-25%[21,22].
Adenoviruses can bind efficiently to epithelial cells and efficiently transfer genes into both replicating and non-replicating cells, although the transfection efficiency varies depending on cell type. Therapy with BMSCs containing Ad/hIL-2 has potential advantages for gene delivery in vivo due to their ability to home to specific organs and to secrete IL-2 into the local microenvironment. This may enhance cytotoxicity against tumor cells while reducing side effects.
Using BMSCs containing Ad/Laz ((β-galactosidase gene), we observed strong positive X-gal staining in the bone marrow, spleen, liver, lungs and kidneys. The transduction efficiency of these BMSCs was 70% at 50 MOI and 90% at 100 MOI. At 50 MOI, BMSCs containing Ad/hIL-2 showed effective anti-tumor effects. These animals showed no evidence of the disease, whereas the control animals developed widespread metastases during the 12 wk observation period. In addition, hamsters treated with BMSCs containing Ad/hIL-2 showed no evidence of side effects until the time of sacrifice. These findings suggest that BMSCs containing Ad/hIL-2 can implant into various organs and that locally-transplanted IL-2 stimulates and activates T4 and T8 cells leading to an attack on tumor cells via paracrine effects, thus killing tumor cells at metastatic sites.
One limitation of this study is that BMSCs were infused prior to the formation of measurable tumor mass in hamsters, thus not allowing us to determine whether the effect of IL-2 was dependent on tumor burden. However, given that BMSCs containing Ad/hIL-2 could suppress cholangiocarcinoma development in hamsters, this therapy may be effective as an adjuvant treatment after curative resection in minimizing residual disease after debulking surgery. These findings suggest that treatment with BMSCs containing Ad/hIL-2 may be one of the potential modalities for cholangiocarcinoma, especially for eradicating residual metastatic biliary cancer.
The authors thank Dr. Tajima for providing the KIGB-5 cholangiocarcinoma cell line.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 1: epidemiology and etiology. J Gastroenterol Hepatol. 2002;17:1049-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 682] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 3. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473; discussion 473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 865] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 4. | Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1493-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Farley DR, Weaver AL, Nagorney DM. "Natural history" of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 164] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Ellis PA, Norman A, Hill A, O'Brien ME, Nicolson M, Hickish T, Cunningham D. Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer. 1995;31A:1594-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Jones DV, Lozano R, Hoque A, Markowitz A, Patt YZ. Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol. 1996;14:2306-2310. [PubMed] |
| 8. | Patt YZ, Jones DV, Hoque A, Lozano R, Markowitz A, Raijman I, Lynch P, Charnsangavej C. Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol. 1996;14:2311-2315. [PubMed] |
| 9. | Sanz-Altamira PM, Ferrante K, Jenkins RL, Lewis WD, Huberman MS, Stuart KE. A phase II trial of 5-fluorouracil, leucovorin, and carboplatin in patients with unresectable biliary tree carcinoma. Cancer. 1998;82:2321-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Pitt HA, Nakeeb A, Abrams RA, Coleman J, Piantadosi S, Yeo CJ, Lillemore KD, Cameron JL. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221:788-797; discussion 797-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 212] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Verbeek PC, Van Leeuwen DJ, Van Der Heyde MN, Gonzalez Gonzalez D. Does additive radiotherapy after hilar resection improve survival of cholangiocarcinoma An analysis in sixty-four patients. Ann Chir. 1991;45:350-354. [PubMed] |
| 12. | Ding L, Lu S, Batchu R, III RS, Munshi N. Bone marrow stromal cells as a vehicle for gene transfer. Gene Ther. 1999;6:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 369] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Clark BR, Keating A. Biology of bone marrow stroma. Ann N Y Acad Sci. 1995;770:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15197] [Article Influence: 584.5] [Reference Citation Analysis (0)] |
| 16. | Kim SW, Kim HJ, Kim SB, Suh C, Shin JS, Park JS, Gong G, Lee JS, Kim SH. Murine bone marrow stromal cells: implications for their use in gene modified cell therapy. Leuk Lymphoma. 2003;44:1973-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp Hematol. 2000;28:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Bonnet MC, Tartaglia J, Verdier F, Kourilsky P, Lindberg A, Klein M, Moingeon P. Recombinant viruses as a tool for therapeutic vaccination against human cancers. Immunol Lett. 2000;74:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Jan YY, Jeng LB, Hwang TL, Wang CS, Chen MF, Chen TJ. Factors influencing survival after hepatectomy for peripheral cholangiocarcinoma. Hepatogastroenterology. 1996;43:614-619. [PubMed] |
| 20. | Berdah SV, Delpero JR, Garcia S, Hardwigsen J, Le Treut YP. A western surgical experience of peripheral cholangiocarcinoma. Br J Surg. 1996;83:1517-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Ju DW, Wang BM, Cao X. Adenovirus-mediated combined suicide gene and interleukin-2 gene therapy for the treatment of established tumor and induction of antitumor immunity. J Cancer Res Clin Oncol. 1998;124:683-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 699] [Article Influence: 22.5] [Reference Citation Analysis (0)] |