Published online Mar 28, 2006. doi: 10.3748/wjg.v12.i12.1859
Revised: October 2, 2005
Accepted: October 26, 2005
Published online: March 28, 2006
AIM: To investigate whether Src, JAK2 and phosphatidylinositol 3-kinase (PI3K) pathways are involved in the proliferation of human colonic tumour cells induced by glycine-extended gastrin (G-gly), the precursor of the mature amidated gastrin and to elucidate the molecular interaction between these three kinases in response to this peptide.
METHODS: Using the human colonic tumour cell line HCT116 as a model, we first measured the activation of PI3K, p60-Src and JAK2 in response to G-gly by in vitro kinase assays. Then we investigated the involvement of these kinases in G-gly-induced cell proliferation by MTT test.
RESULTS: G-gly stimulation induced p60-Src, JAK2 and PI3K activation in HCT116. The different pathways were involved in proliferation of human colon cancer cells induced by G-gly. Furthermore, we found that both Src and JAK2 were necessary to PI3K regulation by this peptide. However, we did not find any cross-talk between the two tyrosine kinases.
CONCLUSION: Our results suggest that the p60-Src/PI3K and JAK2/PI3K pathways act independently to mediate G-gly proliferative effect on human colonic tumour cells.
- Citation: Ferrand A, Kowalski-Chauvel A, Pannequin J, Bertrand C, Fourmy D, Dufresne M, Seva C. Glycine-extended gastrin activates two independent tyrosine-kinases in upstream of p85/p110 phosphatidylinositol 3-kinase in human colonic tumour cells. World J Gastroenterol 2006; 12(12): 1859-1864
- URL: https://www.wjgnet.com/1007-9327/full/v12/i12/1859.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i12.1859
Hyperproliferation of the colonic mucosa is associated with an increased risk of tumour development, and corresponds to an early stage in the adenoma-carcinoma sequence. It is now well established that glycine-extended gastrin (G-gly), the precursor of the mature amidated gastrin, plays an important role in colonic mucosa hyperproliferation. Proliferative effects of G-gly were first described in a pancreatic tumour cell line, AR4-2J[1]. Afterwards, numerous studies have confirmed its mitogenic effects, especially on colonic mucosa, and in vitro studies have shown that G-gly is a growth factor for non transformed cell lines from colon origin or human colon cancer cells[2-5]. Trophic effects of G-gly have also been confirmed in vivo and MTI/G-gly transgenic mice overexpressing G-gly, as well as gastrin-deficient mice perfused with this peptide, display hyperproliferation of the colonic mucosa[6]. Furthermore, perfusion of G-gly into rats results in proliferation of colonic mucosal cells forming aberrant crypt foci and increases the sensitivity to azoxymethane, a colon carcinogen[7].
The p85/p110 PI3K is a lipid kinase composed of two constitutively associated subunits: p85, the regulatory subunit; and p110, the catalytic subunit. Upon stimulation, PI3K phosphorylates the D3 position of phosphoinositides leading to second messengers, namely phosphatidylinositol 3, 4 biphosphate and phosphatidylinositol 3, 4, 5 triphosphate[8]. The PI3K pathway is involved in the regulation of many cellular processes including proliferation and survival. During the last years, many studies have shown the implication of the PI3K in colon carcinogenesis. In particular, the PI3K has been found to play an important role in colon cancer development and progression by promoting cell growth and allowing cells to escape apoptosis[9]. Activation of this pathway is also involved in the progression of human colon adenocarcinoma[10].
Different groups including ours, have demonstrated the involvement of the phosphatidylinositol 3-kinase in G-gly functions. We have reported the activation of lipid kinase in response to this peptide in a pancreatic tumoral cell line, AR4-2J[11]. Afterwards, Hollande et al[12] have described the involvement of the phosphatidylinositol 3-kinase in adhesion and migration of gastric epithelial cells regulated by G-gly. More recently, we described the overexpression of the regulatory subunit p85 as well as an overactivation of the downstream effector Akt, in the hyperproliferative colonic mucosal epithelium of MTI/G-Gly mice[13]. Moreover, in the same study, we have demonstrated the involvement of the PI3K pathway as well as Src and JAK2 pathways in the proliferation of isolated normal murine colonic epithelial cells in response to G-gly.
Our aim here was to investigate whether these three kinases are involved in G-gly-induced proliferation of human colonic tumour cells and to elucidate the molecular interaction between Src, JAK2 and PI3K in response to the peptide. In HCT116, a human colon tumour cell line, we first measured the activation of these kinases by G-gly as well as their involvement in G-gly-induced cells proliferation. The results indicate that both p60-Src and JAK2 are necessary to PI3K regulation by the peptide. However, we did not observe any cross-talk between the two tyrosine kinases, suggesting that the p60-Src/PI3K and JAK2/PI3K pathways act independently.
Polyclonal anti-JAK2 antibody was purchased from Upstate Biotechnology Inc. Monoclonal anti-p60-Src antibody was obtained from Oncogene Science. Rabbit polyclonal antibody specific to p85, the regulatory subunit of the phosphatidylinositol 3-kinase, was kindly provided by Drs. Y. Le Marchand-Brustel and J. F. Tanti (Nice, France). DFO, LY 294002, PP2 and AG490 were from Calbiochem. [γ-32P] ATP (7000 Ci/mmol) was from ICN. Phosphatidylinositol was purchased from Sigma.
HCT116, human colonic tumour cells, were grown in DMEM containing 4.5g/L glucose-glutamax, supplemented with 10% fetal calf serum at 37 °C in a 50mL/L CO2 atmosphere.
Approximately 75 000 cells/well were plated into 96-well plates. Forty-eight hours after plating, cells were treated for 48 h with G-gly (10 pmol/L) with or without inhibitors (10µmol/L). MTT colorimetric assay (MTT, Sigma) was used to measure proliferation as previously described[13].
HCT116 cells were serum-starved for 18 h before peptide addition. After stimulation, the cells were lysed, and the soluble fractions containing identical levels of proteins were immunoprecipitated and analyzed by Western-blot with the indicated antibodies as described previously[14]. Band intensity was analyzed using the image analyzer Biocom (France).
Src kinase activities were determined on anti-p60-Src-immunoprecipitates as previously described[15]. Briefly, kinase assay was carried out at 37 °C for 10 min in kinase buffer (12.5 mmol/L MnCl2, 1.25 mmol/L DTT, 25 mmol/L Hepes, pH 7.4) containing 3.75 µmol/L ATP, 10 µCi/point ATP[γ-32P]. Proteins were separated by SDS–PAGE and the gel was autoradiographied. The gels were then treated with 1N KOH at 50 °C for 1h and autoradiographied. Band intensity was analyzed using the image analyzer Biocom (France).
JAK2 kinase activities were determined on anti-JAK2-immunoprecipitates as previously described[14]. Briefly, kinase assay was carried out at 37 °C for 10 min in kinase buffer (10 mmol/L MnCl2, 5 mmol/L MgCl2, 0.1 mmol/L Vn2+, 10 mmol/L Tris, pH 7.4) containing 3.75 µmol/L ATP, 10 µCi/point ATP[γ-32P]. Proteins were separated by SDS–PAGE and the gel was autoradiographed. Band intensity was analyzed using the image analyzer Biocom (France).
Cell lysates were immunoprecipitated with the indicated antibodies. PI3K assay was performed on immunoprecipitates as described previously[16]. Briefly, phosphatidylinositol used as an exogenous substrate, was prepared in 5 mmol/L HEPES and then sonicated for 15 min at 4 °C. The samples were incubated for 15 min at room temperature with phosphatidylinositol (0.2 mg/mL) and 50 µmol/L ATP[γ-32P] (10 µCi/point). The reaction was stopped by adding HCl 4N and lipids were extracted using agitation in chloroforme/methanol (1/1) solution for 45 s. After centrifugation, the upper phase containing the lipids was analyzed. The phospholipids were separated by thin layer chromatography and analyzed by autoradiography. Spot intensity was analyzed using the image analyzer Biocom (France). When indicated, the cells were pretreated for 1h with 1µmol/L desferrioxamine (DFO).
Statistical analysis was carried out by Student’s t test using GraphPad Prism.
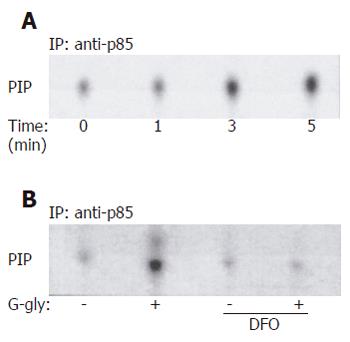
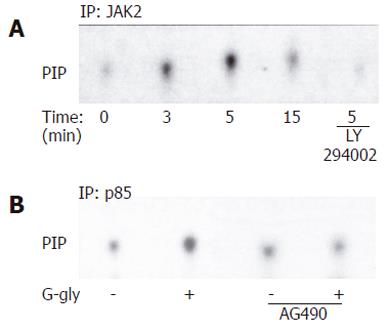
We first tested whether the p85/p110 phosphatidylinositol 3-kinase (PI3K) was activated in HCT116 cells after G-gly stimulation. Cells were treated with the peptide for varying lengths of time. Lipid kinase assay was performed in vitro on anti-p85 immunoprecipitates using inositol phosphate as an exogenous substrate. Lipids were then separated by thin layer chromatography. Increase in phosphatidylinositol phosphorylation was detected within 3-5 min after treatment with G-gly. This stimulation similar to our previous observation in pancreatic tumour cells[11], indicated that PI3K activation was also an early event of G-gly signalling in colon cancer cells. The ferric ion chelator, desferrioxamine (DFO) could inhibit both the binding of G-gly to its receptor and biological activity[17]. To test the specificity of the activation by G-gly, we performed PI3K assays after pretreatment of the cells with DF0 for 1h. DFO could completely inhibit PI3K activation in response to the peptide (Figure 1).
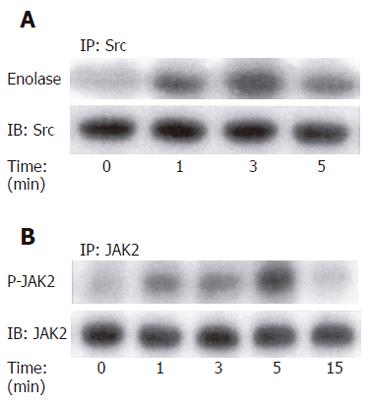
To investigate whether glycine-extended gastrin could regulate p60-Src activation, serum-starved HCT116 cells were treated with G-gly for various times and lysed. Tyrosine kinase assays were performed in anti-p60-Src immunoprecipitates using enolase as an exogenous substrate. Our results indicated that p60-src was very rapidly and transiently activated in HCT116 cells in response to G-gly. The activation of p60-Src was detected 1 min after peptide addition. The stimulation was maximal at 3 min and decreased after 5 min (Figure 2A).
To test whether JAK2 was activated by G-gly, lysates from cells stimulated for various times were immunoprecipitated with anti-JAK2 antibodies and in vitro kinase assays were performed on immunoprecipitates as described in “Methods”. We observed a rapid and transient increase in JAK2 autophosphorylation in response to G-gly stimulation. The maximal activation obtained within 5 min after peptide addition decreased toward the basal level at 15 min (Figure 2B).
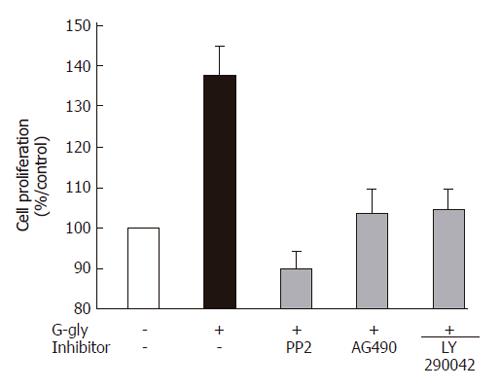
In order to identify the role of PI3K, Src and JAK2 pathways in the proliferation of human colonic tumour cells induced by G-gly, we measured HCT116 proliferation in the presence or absence of specific inhibitors for each pathway, LY290042, PP2 and AG490 respectively. Stimulation of HCT116 cells for 48h by G-gly induced a significant increase of cell proliferation (mean ± SE; 1.38 ± 0.07; P<0.05; n = 5) (Figure 3). Treatment of the cells with PP2, AG 490 or LY 290042 completely inhibited G-gly-induced HCT116 proliferation while inhibitors alone did not affect basal cell proliferation (data not shown). The result indicated that these three pathways could mediate G-gly proliferative effects not only on normal colonic epithelial cells but also on human colonic tumoral cells.
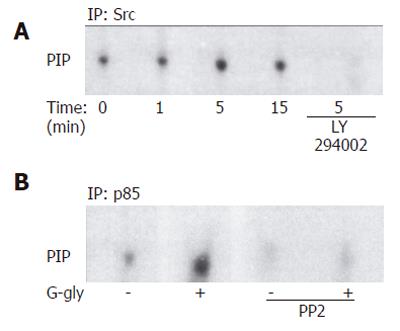
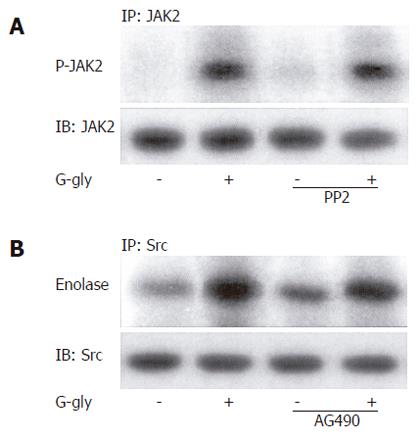
In order to determine whether p60-Src was involved in PI3K activation by G-gly, we first tested whether PI3K activity was detected in association with p60-Src. After peptide treatment for various times, PI3K assays were carried out on anti-p60-Src immunoprecipitates. In response to G-gly, we observed an increased PI-kinase activity coprecipitated with maximal p60-Src after 5 min of peptide stimulation (Figure 4A). To test the PI3K specificity, we added the PI3K inhibitor, LY 294002, in the in vitro lipid kinase assay. Under these conditions, G-gly induced-PI-kinase activity was completely blocked. These results demonstrated that glycine-extended gastrin was associated with p60-Src and PI3K. In order to confirm that an Src family member could act upstream of the PI3K pathway activated by G-gly, we pretreated the cells for 1h with PP2 before G-gly stimulation. PI3K activation was inhibited in PP2 pretreated cells, indicating the role of Src in PI3K activation (Figure 4B).
We then examined the role of JAK2 in PI3K activation by G-gly. After peptide treatment for the time indicated, the cells were lysed and PI3K assays were performed on anti-JAK2 immunoprecipitates. Increased PI-kinase activity was associated with JAK2, detectable at 1 min and maximal after 5 min of peptide stimulation (Figure 5A). The inhibition of PI-kinase activity observed when LY 294002 was added in the in vitro lipid kinase assay indicated that glycine-extended gastrin was associated with JAK2 and PI3K. To confirm the involvement of JAK2 upstream of the G-gly-induced PI3K pathway, the cells were pretreated for 1h with AG 490 prior to G-gly addition. The PI3K activation in response to G-gly was inhibited in the presence of JAK2 inhibitor, indicating the role of this tyrosine kinase upstream in PI3K activation (Figure 5B).
To investigate the possible association between p60-Src and JAK2, solubilized proteins were immunoprecipitated with an anti-JAK2 antibody and the precipitates were analyzed by immunoblot using an anti-p60-Src antibody. No p60-Src protein associated with JAK2 was detected in control cells or in cells stimulated with G-gly, whereas comparable amounts of JAK2 proteins were detected in immunoprecipitates (data not shown).
Independently of any association, we studied whether Src-family kinases were involved in JAK2 activation by G-gly. We tested the effect of PP2 on JAK2 activity. Cells were pretreated for 1h with PP2 5 min before stimulation with G-gly. JAK2 kinase assay was performed on JAK2 immunoprecipitates. PP2 did not inhibit G-gly-induced JAK2 activation, indicating that Src kinases were not involved in upstream of JAK2 activation by G-gly (Figure 6A). Similarly, using AG490, we tested a possible role of JAK2 upstream in p60-Src activation. The presence of AG490 in the incubation medium did not significantly change p60-Src activation by G-gly (Figure 6B). Indeed, in this human colon tumoral cell line, activation of the two tyrosine-kinases, p60-Src and JAK2, in response to G-gly was entirely independent.
The p85/p110 phosphatidylinositol 3-kinase (PI3K) is a lipid kinase which plays an important role in human colon cancer. Its specific inhibitor, LY 294002, has been shown to inhibit cell growth and to induce apoptosis. Treatment of human colon cancer cell lines in vitro with LY 294002 or transplanted in vivo abolishes tumour cell growth and leads to apoptosis[9]. In addition, Philp et al[18] have identified somatic mutations in the gene of the p85 subunit leading to a constitutive active form of PI3K. These mutations are present in primary human colon tumours and cancer cells, suggesting that PI3K is involved in human colonic tumorigenesis
Non-amidated gastrin precursors, including G-gly, are produced by colorectal cancers and exert growth factor effects on these tissues[19,20]. They are expressed in 80% - 90% of colorectal tumours and polyps in human beings[21-24]. Identification of signalling pathways involved in colonic mucosa hyperproliferation is important for the understanding of tumour processes. In a previous study we have identified that Src, JAK2 and PI3K pathways are overactivated in the colonic epithelium of mice overexpressing G-gly and involved in G-gly-induced proliferation of normal colonic epithelial cells isolated from control mice[13].
In the current study, we used the human colon tumour cell line, HCT116, to investigate whether these three kinases interact to regulate the growth of human tumoral colon cells. The results indicate that both p60-Src and JAK2 are necessary to PI3K regulation by the peptide. Theses results are in accordance with previous reports showing that JAK2 are involved upstream of the PI3K pathway of other cellular models. Pretreatment of human neutrophils with the JAK2 inhibitor, AG 490 could abolish the stimulation of the p85/p110 PI3K in response of the granulocyte-macrophage colony-stimulating factor. In addition, Attoub et al[25] have shown that leptin can induce a transient elevation of the PI3K lipid products in JAK2 immunoprecipitates prepared from MDCK cells. Similarly, Src family kinases are involved upstream of the PI3K/AKT pathway[26,27]. Previous studies suggest that the IRS proteins could serve as scaffolding intermediates between tyrosine kinases and PI3K[28]. This mechanism is likely involved in PI3K activation by G-gly. In HCT116 cells we observed a basal association between JAK2 and IRS1 and the tyrosine phosphorylation of IRS-1 in response to G-gly (data not shown). In addition, we have previously reported that tyrosine phosphorylation of IRS-1 in pancreatic tumour cells, leading to the rapid recruitment of the regulatory subunit of PI3K, might represent a common mechanism for PI3K activation by different factors including insulin and G-gly[11]. Obviously, we cannot exclude other hypotheses such as interaction between the SH2 domain of p85 and phospho-tyrosine of p60-Src or JAK2. Different studies have previously described the involvement of Src family kinases upstream of JAK2 activation. For example, cell transformation by the oncogene v-Src[29] or the overexpression of certain members of the Src family-kinases as Lck[30] can lead to a constitutive activation of JAK2. However, we did not find any cross-talk between the two tyrosine kinases, suggesting that the p60-Src/PI3K and JAK2/PI3K pathways act independently. The involvement of two different mechanisms upstream of the lipid kinase might be a way to amplify the PI3K signal. However, in our study, the level of PI3K activity associated with JAK2 or p60-Src was not different from that observed in anti-p85 immunoprecipitates. As for many other signalling molecules, it seems that the cellular localization of PI3K is important for the function of the enzyme. Therefore the recruitment of PI3K to different cell compartments by the two independent tyrosine-kinases allows the enzyme to play a complementary role. The finding that p60-Src and JAK2 do not associate in response to G-gly stimulation, demonstrates that the p60-Src/PI3K and JAK2/PI3K pathways act independently and indicates that they might be involved in different cell compartments. The analysis of the cellular localization of phosphatidylinositol 3-kinase, might be crucial to understand the role of this signalling molecule in different biological effects of G-gly.
Our previous studies indicate that gastrin precursors contribute to the initiation phases of colon carcinogenesis by upregulating the signalling pathways involved in cellular proliferation and cell survival. The results of this study demonstrate that G-gly is also able to regulate proliferation of tumoral colonic cells and support the idea that gastrin precursors are not only involved in the initiation steps of the colon tumour process but also involved in the later stages of tumour progression after genetic alterations, by conferring a growth advantage to the cells via the constitutive activation of numerous signalling pathways.
S- Editor Wang J L- Editor Wang XL E- Editor Liu WF
| 1. | Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994;265:410-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 247] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Singh P, Owlia A, Espeijo R, Dai B. Novel gastrin receptors mediate mitogenic effects of gastrin and processing intermediates of gastrin on Swiss 3T3 fibroblasts. Absence of detectable cholecystokinin (CCK)-A and CCK-B receptors. J Biol Chem. 1995;270:8429-8438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Iwase K, Evers BM, Hellmich MR, Guo YS, Higashide S, Kim HJ, Townsend CM. Regulation of growth of human gastric cancer by gastrin and glycine-extended progastrin. Gastroenterology. 1997;113:782-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Stepan VM, Sawada M, Todisco A, Dickinson CJ. Glycine-extended gastrin exerts growth-promoting effects on human colon cancer cells. Mol Med. 1999;5:147-159. [PubMed] |
| 6. | Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin(17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer. 2001;94:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Duronio V, Scheid MP, Ettinger S. Downstream signalling events regulated by phosphatidylinositol 3-kinase activity. Cell Signal. 1998;10:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Semba S, Itoh N, Ito M, Harada M, Yamakawa M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3'-kinase, in human colon cancer cells. Clin Cancer Res. 2002;8:1957-1963. [PubMed] |
| 10. | Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Tyrosine phosphorylation of insulin receptor substrate-1 and activation of the PI-3-kinase pathway by glycine-extended gastrin precursors. Biochem Biophys Res Commun. 1997;236:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Hollande F, Choquet A, Blanc EM, Lee DJ, Bali JP, Baldwin GS. Involvement of phosphatidylinositol 3-kinase and mitogen-activated protein kinases in glycine-extended gastrin-induced dissociation and migration of gastric epithelial cells. J Biol Chem. 2001;276:40402-40410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Ferrand A, Bertrand C, Portolan G, Cui G, Carlson J, Pradayrol L, Fourmy D, Dufresne M, Wang TC, Seva C. Signaling pathways associated with colonic mucosa hyperproliferation in mice overexpressing gastrin precursors. Cancer Res. 2005;65:2770-2777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Ferrand A, Kowalski-Chauvel A, Bertrand C, Pradayrol L, Fourmy D, Dufresne M, Seva C. Involvement of JAK2 upstream of the PI 3-kinase in cell-cell adhesion regulation by gastrin. Exp Cell Res. 2004;301:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657-20663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Gastrin stimulates tyrosine phosphorylation of insulin receptor substrate 1 and its association with Grb2 and the phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26356-26361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Pannequin J, Barnham KJ, Hollande F, Shulkes A, Norton RS, Baldwin GS. Ferric ions are essential for the biological activity of the hormone glycine-extended gastrin. J Biol Chem. 2002;277:48602-48609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426-7429. [PubMed] |
| 19. | Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta. 2004;1704:1-10. [PubMed] |
| 20. | Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238:15-29. [PubMed] |
| 21. | Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 22. | Nemeth J, Taylor B, Pauwels S, Varro A, Dockray GJ. Identification of progastrin derived peptides in colorectal carcinoma extracts. Gut. 1993;34:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Kochman ML, DelValle J, Dickinson CJ, Boland CR. Post-translational processing of gastrin in neoplastic human colonic tissues. Biochem Biophys Res Commun. 1992;189:1165-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Smith AM, Watson SA. Gastrin and gastrin receptor activation: an early event in the adenoma-carcinoma sequence. Gut. 2000;47:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, Gespach C. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 2000;14:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 183] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114-130. [PubMed] |
| 27. | Penuel E, Martin GS. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol Biol Cell. 1999;10:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Yamauchi T, Kaburagi Y, Ueki K, Tsuji Y, Stark GR, Kerr IM, Tsushima T, Akanuma Y, Komuro I, Tobe K. Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1, -2, and -3, their association with p85 phosphatidylinositol 3-kinase (PI3-kinase), and concomitantly PI3-kinase activation via JAK2 kinase. J Biol Chem. 1998;273:15719-15726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Murakami Y, Nakano S, Niho Y, Hamasaki N, Izuhara K. Constitutive activation of Jak-2 and Tyk-2 in a v-Src-transformed human gallbladder adenocarcinoma cell line. J Cell Physiol. 1998;175:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |