Published online Mar 14, 2006. doi: 10.3748/wjg.v12.i10.1626
Revised: November 23, 2004
Accepted: December 9, 2004
Published online: March 14, 2006
AIM: To determine the distal intramural spread (DIS) margin of rectal cancer.
METHODS: Sixty-one p53-positive specimens of rectal cancer were used. After conventional hematoxylin and eosin (H&E) staining, the DIS margin of rectal cancer in large specimens was examined by immunohistochemistry. The patients were divided into A, B, C, and D groups. After a long-term follow-up, the survival curves of the four groups were estimated using the life table.
RESULTS: Fifty-one of the sixty-one cases (83.6%) had DIS. The extent of DIS ranged 0.11-3.5 cm; meanwhile the mean of DIS measured by H&E staining was 0.13 cm. The significant difference was found between the means (t=5.622, P<0.0001). Only 1 of 51 patients had DIS greater than 3 cm. The DIS was less than 1.0 cm in most rectal cancer patients. The long-term results indicated that the survival rate of the patients whose DIS was greater than 1.0 cm was lower than that of the patients whose DIS was less than 0.5 cm.
CONCLUSION: Rectal cancer patients with DIS greater than 1.0 cm have poor prognosis.
- Citation: Pan ZZ, Wan DS, Zhang CQ, Shao JY, Li LR, Chen G, Zhou ZW, Wang FL. Using p53-immunostained large specimens to determine the distal intramural spread margin of rectal cancer. World J Gastroenterol 2006; 12(10): 1626-1629
- URL: https://www.wjgnet.com/1007-9327/full/v12/i10/1626.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i10.1626
Defining the optimal distal surgical margin is very important for surgical oncologists during rectal cancer resection[1]. To ensure complete excision of cancer and maximum protection of normal tissue, we should define the length of DIS. Though a distal margin greater than 5 cm is advocated in the past[2], some studies proposed that a distal excision margin of 1 cm is sufficient[3-5], but the molecular clearance margin of DIS is unknown. Molecular techniques have been used to identify tumor markers and occult tumor cells in recent years[6] and the clear margin determined by molecular methods should be different from that by traditional histopathological methods. The accurate assessment of DIS could guide precise surgical resection, aid in estimating prognosis and predict recurrence site, but the molecular margin of DIS of rectal cancer is unknown. In this study, we used p53-immunostained large specimens of rectal cancer to measure the molecular margin of DIS and to clarify the influence of DIS margin on the long-term survival of patients after the resection of rectal cancer.
By using conventional hematoxylin-eosin (H&E) and p53-immunostaining (LSAB), we identified 61 p53-positive cases in 97 rectal cancer patients who were surgically treated in our hospital between August 1996 and October 1997. The characteristics of the 61 patients are listed in Table 1.
| Male | 33 |
| Female | 28 |
| Age (yr) | 54 (22–79) |
| Gross morphology | |
| Fungating | 14 |
| Ulcerative | 46 |
| Infiltrative | 1 |
| Histologic type | |
| Papillary adenocarcinoma | 8 |
| Tubular adenocarcinoma | 52 |
| Mucinous adenocarcinoma | 1 |
| Histologic grade | |
| Well differentiated | 8 |
| Moderately differentiated | 39 |
| Poorly differentiated | 14 |
| Dukes’ stage | |
| A | 19 |
| B | 16 |
| C | 22 |
| D | 4 |
Fresh specimens were opened longitudinally, straightened without stretching and pinned to a cork board. The distal margin (A1) was measured and fixed by 10% formalin for more than a week. The whole specimen including oral and anal edges of the tumor was cut longitudinally into 5-mm-thick giant sections. The sections were embedded in paraffin wax and then serial 8-μm-thick sections were cut from the giant sections on a large microtome. Routine H&E staining and p53-immunostaining (LSAB, 1:100 diluted mouse monoclonal antibody to p53, DO-7; DAKO Ltd, Denmark) were performed respectively. The distal margin was measured on large glass specimens (B1) and the extent of DIS was measured microscopically (b1). The microscopic extent of DIS was converted as the distance of DIS in fresh specimen (a1) by the tissue shrinkage ratio by comparing the distal margin measured in fresh specimens before fixation (A1) to that measured on large sections macroscopically (B1) in each case (A1/B1=a1/b1). The data were converted data.
Both means of the DIS obtained by H&E staining and p53-immunostaining were compared by the paired sample statistic t test. According to the extent of DIS by p53-immunostaining, we divided the patients into four groups: A (DIS = 0.00 cm), B (DIS = 0.01-0.50 cm), C (DIS = 0.51-1.00 cm) and D (DIS > 1.00 cm) .The survival curves of the groups were generated by the life table and compared by Gehan test. SPSS software (8.0) was used for all statistical analyses. P < 0.05 was considered statistically significant.
Under microscope, no cancer cells were found on the edge of resection in the large specimens. By p53 immunostaining, DIS of p53-positive tumor cells was found in normal glandular epithelial tissue next to the tumor in 23 cases under the mucosa away from the tumor in 40 cases. Emboli of cancer cells were found in micro-veins and/or micro-lymph vasculature under the mucosa in 35 cases. Nest formation of cancer cells was found under the mucosa away from the tumor and a clear line between the tumor and the normal tissue was noted in 21 cases. Spread of cancer cells through the above four pathways was observed but there was a clear large space between the metastatic cells and the main tumor.
By H&E staining, no DIS was found in 32 cases, 29 cases had DIS (range: 0.10-1.39 cm, mean: 0.13 cm, 95%CI: 0.1-0.16 cm). By p53 immunostaining, only 10 cases had no DIS, 51 cases had DIS (range: 0.11-3.5 cm, mean: 0.59 cm, 95%CI: 0.5-0.67 cm). There was a significant difference between the means obtained by p53-immunostaining and H&E staining (t = 5.622, P < 0.0001, Table 2).
| DIS | IHC | H&E |
| 0 cm | 10 | 32 |
| 0.01 – 0.50 cm | 26 | 26 |
| 0.51 – 1.00 cm | 14 | 2 |
| >1.00 cm | 11 | 1 |
| x | 0.59 cm | 0.13 cm |
| t | 5.622 | |
| P | <0.0001 | |
By December 2003, the follow-up time ranged 266-2 485 d with a mean of 1 621.4 d. All cases were followed up. Twenty-nine patients died of cancer recurrence. There were 32 disease-free cases in the study.
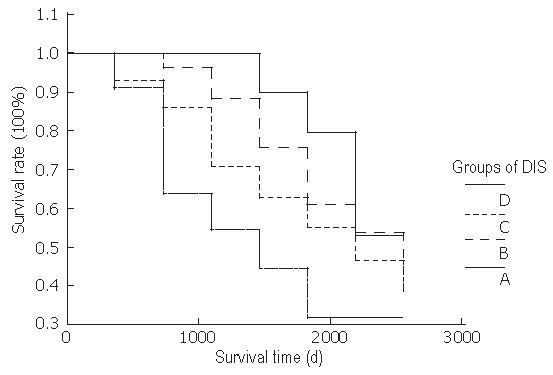
The 5-year survival rate of 61 patients was 57.78%. The 5-year survival rate of groups A, B, C, and D was 79.41%, 60.93%, 55.70%, and 31.88%, respectively. There was no statistical difference among the survival rates of groups A, B, and C. The survival rate of group D was significantly lower than that of groups A and B (Table 3 and Figure 1).
| Group | Gehan value | P |
| A-B | 1.204 | 0.273 |
| A-C | 1.685 | 0.194 |
| A-D | 5.359 | 0.021 |
| B-C | 0.814 | 0.367 |
| B-D | 4.627 | 0.032 |
| C-D | 1.124 | 0.289 |
Different from other studies, the whole specimen was expanded with a similar pull force by surgeons during operation and pinned on a flat board. Then the specimen was fixed by 10% formalin for more than 7 d. The extent of DIS was measured on large specimens microscopically. A tissue shrinkage ratio between the distance measured in fresh specimens from surgical margin to distal tumor edge was used to convert the extent of DIS microscopically to the extent of DIS in situ[7]. The error of DIS measured microscopically caused by the shrinkage of tissue in formalin may be avoided by the above method. Moreover, missed diagnosis of DIS should be reduced because the continuous intramural spread can be observed on large specimens.
Mutation of p53 gene is a late event of colorectal carcinogenesis[8]. Using molecular biological techniques, Brennan et al[9] and Hayashi et al[10] assessed the p53 gene mutation in clearance margins of 25 primary squamous cell carcinomas of the head and neck and local lymph nodes of 120 colorectal cancers. p53 gene mutation was found in some specimens without tumor residue, 40-70% patients with p53 gene mutation in clearance margins or lymph nodes had local recurrence and regional lymph node metastasis after the operation. No metastasis occurred in patients without p53 gene mutation. Their studies indicate that molecular biological analysis is more sensitive in finding hidden tumor cells by identifying tumor markers and can predict the site of local recurrence and the prognosis of patients.
Mutation of p53 gene results in the accumulation of p53 protein and p53 protein is regarded as a useful tumor marker[11]. p53-positive tumor cells that cannot be detected by H&E staining can be identified by immunohistochemical staining. Furthermore, hidden tumor cells usually are too small to be found by microscopy after conventional H&E staining.
In our study, we compared the extent of DIS by p53-immunostaining to that by H&E staining. The results between these two methods were similar, when DIS was less than 0.5 cm. In some cases, when DIS was greater than 0.5 cm, spread of single satellite cells in normal tissue was difficult to be identified by H&E staining. Therefore, H&E staining tends to cause false negative results compared to immune method. Because immunostaining is more sensitive than H&E staining, more positive diagnoses were made. The mean of DIS results was also higher by immunostaining (P<0.05, Table 2.).
Accurate identification of DIS of rectal cancer is very important for determining the clearance margin during the operation and preventing anastomosis recurrence[12]. According to the literatures published, DIS detected by H&E staining usually exists in 14.5-65.6% rectal cancer patients[3,7,8]. Because the results of DIS in rectal cancer are different in different studies[13], there is no universal agreement on the length of distal normal rectum that should be removed during radical resection[14]. Some authors suggested that 1-2 cm of distal removal of normal rectum is enough[4]. On the other hand, some cellular and molecular studies suggested that 2 cm is not safe[9]. In our study, though 83.6% (51/61) of the patients had DIS, the length of DIS in 78.4% (40/51) was less than 1 cm, in 6% (3/51) greater than 2 cm and only in 1.6% (1/51) greater than 3 cm, suggesting that 2 or 3 cm of the distal clearance margin is safe enough for most (95% or above) rectal cancers during radical resection.
It was reported that DIS is correlated to depth of cancer invasion and distal metastasis. Patients with positive DIS have a higher risk for developing metastasis after radical resection and their disease-free survival rate is lower than that of those with negative DIS[10]. In our study, patients with longer DIS had a lower survival rate (Figure 1). If DIS is greater than 1 cm, patient survival rate is markedly decreased regardless of distal normal rectum excision and negative tumor residue in distal surgical edge. The relationship between DIS and survival rate indicates that DIS is a prognostic factor of rectal cancer[15].
S- Editor Guo SY L- Editor Wang XL E- Editor Liu WF
| 1. | Zaheer S, Pemberton JH, Farouk R, Dozois RR, Wolff BG, Ilstrup D. Surgical treatment of adenocarcinoma of the rectum. Ann Surg. 1998;227:800-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | GOLIGHER JC, DUKES CE, BUSSEY HJ. Local recurrences after sphincter saving excisions for carcinoma of the rectum and rectosigmoid. Br J Surg. 1951;39:199-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 277] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Vernava AM, Moran M, Rothenberger DA, Wong WD. A prospective evaluation of distal margins in carcinoma of the rectum. Surg Gynecol Obstet. 1992;175:333-336. [PubMed] |
| 4. | Shirouzu K, Isomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter-preserving surgery. Cancer. 1995;76:388-392. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Moore HG, Riedel E, Minsky BD, Saltz L, Paty P, Wong D, Cohen AM, Guillem JG. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Takayama O, Yamamoto H, Ikeda K, Ishida H, Kato T, Okuyama M, Kanou T, Fukunaga M, Tominaga S, Morita S. Application of RT-PCR to clinical diagnosis of micrometastasis of colorectal cancer: A translational research study. Int J Oncol. 2004;25:597-604. [PubMed] |
| 7. | Søndenaa K, Kjellevold KH. A prospective study of the length of the distal margin after low anterior resection for rectal cancer. Int J Colorectal Dis. 1990;5:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Baker SJ, Preisinger AC, Jessup JM, Paraskeva C, Markowitz S, Willson JK, Hamilton S, Vogelstein B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990;50:7717-7722. [PubMed] |
| 9. | Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch WM, Goodman SN, Sidransky D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 497] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Hayashi N, Ito I, Yanagisawa A, Kato Y, Nakamori S, Imaoka S, Watanabe H, Ogawa M, Nakamura Y. Genetic diagnosis of lymph-node metastasis in colorectal cancer. Lancet. 1995;345:1257-1259. [RCA] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 192] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Mak T, Lalloo F, Evans DG, Hill J. Molecular stool screening for colorectal cancer. Br J Surg. 2004;91:790-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Hall NR, Finan PJ, al-Jaberi T, Tsang CS, Brown SR, Dixon MF, Quirke P. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent. Predictor of survival but not local recurrence? Dis Colon Rectum. 1998;41:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 221] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Kwok SP, Lau WY, Leung KL, Liew CT, Li AK. Prospective analysis of the distal margin of clearance in anterior resection for rectal carcinoma. Br J Surg. 1996;83:969-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Leong AF. Selective total mesorectal excision for rectal cancer. Dis Colon Rectum. 2000;43:1237-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Ono C, Yoshinaga K, Enomoto M, Sugihara K. Discontinuous rectal cancer spread in the mesorectum and the optimal distal clearance margin in situ. Dis Colon Rectum. 2002;45:744-79; discussion 744-79;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |