INTRODUCTION
Pancreatic cancer is the fifth most common cause of cancer death in Japan[1]. It is estimated that there are 20000 cases every year, which is similar to the number of deaths from this disease. The reasons for its very high mortality rate include the lack of early diagnosis, a low resectability at the time of initial diagnosis, and the rapid recurrence after resection. Surgery is rarely a curative option in pancreatic cancers because of local extension and metastasis. Chemotherapy for advanced pancreatic cancer is palliative. The use of 5-FU in combination with radiation in the locally advanced setting has been shown to enhance survival. In a randomized trial between gemcitabine and 5-FU, gemcitabine showed significantly better results in terms of the clinical benefit effect and survival[2].
Gemcitabine (2′, 2′-difluorodeoxycitidine) is a nucleoside analogue well known for its antitumor activity in several solid tumors[3]. It is one of the drugs effective for pancreatic cancers in the clinical treatment[4]. Various general mechanisms of gemcitabine have been described[5-7]. A primary mechanism of gemcitabine is the blocking of DNA synthesis by inhibiting DNA polymerase activity[8]. Gemcitabine itself is not active until it enters the cells. Its intracellular transport is mediated by facilitated diffusion[9]. Intracellularly, gemcitabine is phosphorylated to its active metabolites by deoxycitidine kinase to difluorodeoxycitidine monophosphate (dfdCMP), difluorodeoxycitidine diphosphate (dfdCDP) and difluorodeoxycitidine triphosphate (dfdCTP). All three of the metabolites interfere at different steps in the processing of DNA. The dfdCTP is incorporated into DNA and as such can obstruct DNA replication and repair. The dfdCTP can also be incorporated into RNA and can inhibit CTP-synthetase. The dfdCMP can also inhibit dCMP-deaminase. The dfdCDP is an inhibitor of ribonucleotide reductase, and its action was shown to lead to the depletion of the DNA precursor pool, dNTP. Inhibition of DNA synthesis leads to growth inhibition or cell death. In previous studies, gemcitabine has been shown to play a major role in the apoptosis of certain tumor cell lines[10-12]. There are several pathways that relate to gemcitabine-induced apoptosis. Nicole et al[13] reported that gemcitabine-mediated apoptosis is caspase-dependent in pancreatic cancers; Jones et al[14] showed that gemcitabine-induced apoptosis is achieved through the blocking of NF-kB in non-small cell lung cancer cells (NSCLC). In addition, gemcitabine-induced apoptosis is also related to signal-regulated kinase (ERK), Akt, Bcl-2 and p38 mitogen-activated protein kinase (MAPK) pathways[15-17]. Achiwa et al[18] indicated that the increase in human equilibrate nucleoside transport (hENT) expression is a determinant of gemcitabine sensitivity in NSCLC cells. However, the molecular mechanism of gemcitabine-induced apoptosis has not been fully elucidated.
Pancreatitis-associated protein (PAP) is a secretory protein of pancreatic acinar cells. It is almost absent in the normal pancreas, but is induced in acute and chronic pancreatitis. We have reported the expression of PAP mRNA in cancer tissues and have measured PAP levels in the sera and pancreatic juice of patients with gastrointestinal cancers[19-21]. We found that serum PAP levels were increased in 40% of patients with pancreatic cancer. We also reported that PAP levels in endoscopically aspirated pancreatic juice were positive in 55% of pancreatic cancers. PAP levels were significantly higher in both the serum and pancreatic juice in cases of pancreatic cancer, compared to chronic pancreatitis. Cytokines such as tumor necrosis factor-α, interferon-γ, and interleukin-6 induce PAP mRNA expression in the pancreatic acinar AR4-2J cell line. We found that the enhanced expression of PAP in pancreatic adenocarcinoma is caused by both ectopic expression in cancer cells and induction in acinar cells[22].
TP53INP1[23] was previously called stress-induced protein (SIP)[24] or p53-dependent inducible nuclear protein (p53DINP1)[25]. TP53INP1 is strongly induced in acinar cells during acute pancreatitis in mice, and is also overexpressed in response to various stresses in vitro. TP53INP1 gene expression is wild-type p53-dependent[26]. There is a functional p53-response element within the promoter region of the TP53INP1 gene, and TP53INP1 mRNA expression is activated in cells expressing wild-type p53 in response to various stresses. One of the major functions of TP53INP1 is promoting cellular apoptosis. Glycogen synthase kinase 3β (GSK-3β) is a multifunctional serine/threonine kinase mediating various cellular signaling pathways. The particular pathway depends on its substrates for phosphorylation[27]. Since GSK-3β is also an important mediator of an apoptotic signal, it is plausible that the GSK-3β deregulation observed in cancer cells confers resistance to chemotherapy, which is a major cause of treatment failure in human cancers[28].
In this study we investigated the effect of gemcitabine on the PANC-1 cells in terms of apoptosis-related factors.
MATERIALS AND METHODS
Cell culture and gemcitabine treatment
A human pancreatic cancer cell line, PANC-1, obtained from the American Type Culture Collection (ATCC, MD, USA), was maintained in Dulbecco’s modified Eagle's medium supplemented with 100 mL/L fetal calf serum, penicillin, and kanamycin at 37°C in a 50 mL/L CO2, 950 mL/L air atmosphere. Gemcitabine (Eli-Lilly Japan, Kobe, Japan) at a concentration of 50 mg/mL was dissolved in the serum free culture medium and stored at -20 °C in the freezer. The concentration range of the treatment was from 2.5 mg /L to 1 000 mg/L.
Cell growth evaluation
The Alamarblue dye method was used for cell growth analysis. The 1×104 cells were plated in 96-well microtiter plates. After being incubated for 24 h, gemcitabine was added to the medium. Twenty μL of AlamarBlue dye solution (Iwaki Glassware, Inc., Tokyo, Japan) was added to wells containing 200 μL of medium at the time 12, 24, 48, and 72 h. After being incubated for 3 h, the cell growth was evaluated as the absorbance (A) using a spectrophotometer (Dai-Nippon Pharmaceutical Co., Osaka, Japan). An excitation wavelength of 540 nm was used, and the emission was read at 620 nm. The color of AlamarBlue stock is violet, and changes to red when oxidized. Each treatment was applied to 6 wells, and the experiments were repeated 3 times.
DNA fragmentation assay
DNA fragmentation was quantitatively assayed using a DNA fragmentation enzyme-linked immunosorbent assay (ELISA) kit (Boehringer Mannheim GmbH, Mannheim, Germany) according to the protocol. Cells were cultured in flat-bottom, 96-well microplates. After incubation in gemcitabine-supplemented media for 24 h, the cells were detached from the wells. The cells were lysed with lysis buffer, and the lysate was processed for streptavidin-coated microtiter plates. After incubation with biotin-labeled antihistone antibody and peroxidase-labeled anti-DNA antibody, the amount of fragmented DNA was determined using 2, 2′-azino-bis-3-ethylbenzthiazoline-sulfonate (ABTS) as a substrate. The plates were read on a Labsystems integrated EIA (Dai-Nippon Pharmaceutical Co., Osaka, Japan) at wavelengths of 540 nm and 620 nm. Each treatment was added to duplicate wells, and the experiment repeated 3 times.
Total RNA was extracted from pancreatic cancer cells using a SV Total RNA Isolation system kit (Promega, Madison, WI, USA). RNA concentrations were determined with spectrophotometry. RT was performed using a PowerScriptTM Reverse Transcriptase kit (Clontech Laboratories, Inc., Palo Alto, CA, USA). First strand cDNA was synthesized from 8 μg of total RNA at 65 °C for 5 min after RT; the reverse transcriptase was inactivated by incubating at 42 °C for 60 min and the reaction was terminated by heating at 70 °C for 25 min. Then, cycles of amplification were performed on a DNA thermal cycler (Perkin Elmer Cetus, Inc., Norwalk, CT, USA) as follows: PAP primers were denatured at 91°C for 60 sec, annealed at 54 °C for 60 sec, and polymerized at 72 °C for 45 sec. Then, extension was performed at 72 °C for 10 min. TP53INP1 primers were denatured at 94 °C for 50 sec, annealed at 62 °C for 40 sec, and polymerized at 72 °C for 50 sec. The extension was performed at 72 °C for 10 min. The PAP primer pairs were sense: 5’-CTCCTGATTGCCTCCTCAAG -3’ and antisense: 5’-AAACGTACCCTCTCTTTAGG -3’, producing a fragment of 441 bp. TP53INP1 mRNA was specifically amplified with the following primers: sense 5’-CATCCAGCCAAACTCTCAGTC -3’ and antisense 5’-GCGACGAAGGCTATTTCTGT -3’. The size of the fragment was 703 base pairs (bp). The GAPDH of 452 bp was used as an internal control. Eight-microgram aliquots of the RT-PCR products were subjected to electrophoresis on a 20 g/L agarose gel and visualized with SYBR Gold (Molecular Probes, Inc., Eugene, OR, USA) at a 1:10000 dilution in dimethylsulfoxide, and exposed to ultraviolet 312-nm light.
Semiquantitative RT-PCR analysis
The gene expression of PAP and TP53INP1 was semi-quantitatively analyzed with an image analyzer (ATTO Densitograph ver. 3.02, ATTO, Inc., Tokyo, Japan). The relative expression intensity was calculated according to the following formula: PAP and TP53INP1 mRNA in a sample/GAPDH mRNA in a sample.
Western blot analysis
For Western blot analysis, 1×106 PANC-1 cells were lysed in a CelLyticTM mammalia tissue lysis/ extraction reagent buffer (Sigma-Aldrich, St. Louis, MO, USA). Aliquots were boiled for 5 minutes with Laemli buffer, resolved on a 125 g/L SDS-PAGE gel and eletrophoretically transferred to nitrocellulose membrane (Amersham, Buckingham, UK). Membranes were blocked for 1 h in TBS containing 50 g/L dry milk and 1 g/L Tween 20. Membranes were then incubated with either anti-rabbit phospho-GSK-3β (ser9) polyclonal antibody (Cell Signaling Technology, Inc. Beverly, MA, USA) or anti-mouse GSK-3β monoclonal antibody (BD Transduction Laboratories, Lexington, KY, USA) for one night at 4 °C. After incubation with anti-rabbit or anti-mouse IgG second antibody labeled with peroxidase, the membranes were visualized with an ATTO image analyzer.
Statistical analysis
Experimental results were expressed as the mean ± SE. The difference between the means was evaluated with the Mann-Whitney U test. P < 0.05 was considered statistically significant. The statistical analysis was performed using StatView-5.0 (SAS Institute Inc. Tokyo, Japan).
RESULTS
Gemcitabine inhibited cell growth
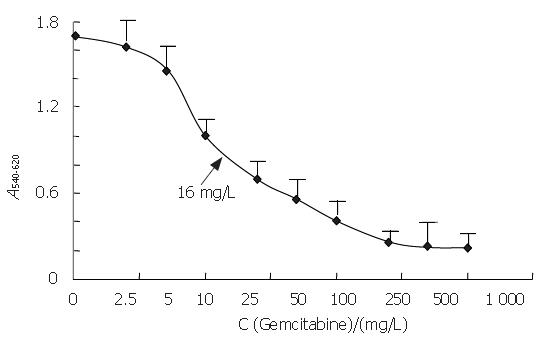
The median 50% inhibitory concentration (IC50) of gemcitabine after a 48-h exposure was 16 mg/L (Figure 1). We also measured the effect of 4, 16 and 64 mg/L gemcitabine at 12, 24, 36, and 48 h. The growth of PANC-1 cells was inhibited by gemcitabine in a concentration-dependent manner (P < 0.0001, Figure 2) and cell growth was also inhibited throughout the time course (P < 0.0001).
Figure 1 Effect of gemcitabine on the growth of PANC-1 cells.
Figure 2 Inhibitory effect of gemcitabine on the growth of PANC-1 cells.
Gemcitabine induced apoptosis
1×104 PANC-1 cells were cultured in flat-bottom, 96-well microplates. After incubation in gemcitabine-supplemented media for 24 h, a DNA fragmentation assay was performed. The concentration of gemcitabine used in the DNA fragmentation assay was 16 mg/L. The DNA fragmentation rate in the gemcitabine-treated group was 44.7%, whereas the fragmentation rate in the untreated group was 25.3%. The fragmentation in the gemcitabine treated group was significantly higher than that in the untreated group (P = 0.0157).
Expression of PAP, TP53INP1 mRNA
We exposed the PANC-1 cells to 4, 16 and 64 mg/L gemcitabine for 48 h. The expression of PAP and TP53INP1 mRNA was assessed using RT-PCR. The PAP mRNA expression was decreased after being treated with gemcitabine, and disappeared after being treated with 16 mg/L gemcitabine. The semiquantitative analysis showed the intensity of 16 mg/L gemcitabine-treated cells to be significantly lower than that of the untreated cells (P = 0.0165, Figure 3). The TP53INP1 mRNA was increased after exposure to gemcitabine. The intensity of the cells treated with 4, 16 and 64 mg/L gemcitabine was 2 times, 8.5 times and 14.6 times higher than in the untreated cells, respectively. The increasing density of TP53INP1 mRNA was concentration-dependent. The P value of the interrelation of the untreated group to the 4 mg/L gemcitabine-treated group, 16 mg/L gemcitabine-treated group and 64mg/L gemcitabine-treated group were 0.0015, 0.0009 and 0.0352, respectively.
Figure 3 PAP mRNA expression after gemcitabine treatment.
Expression of phospho-GSK-3¦Â and GSK-3¦Â proteins
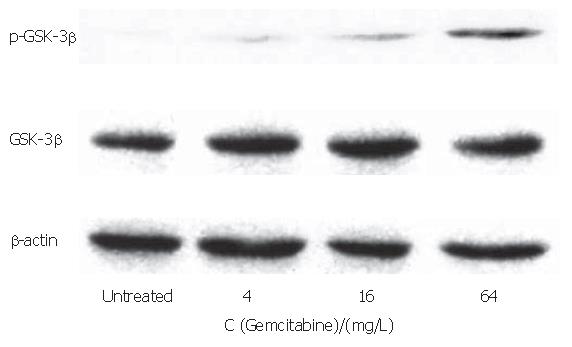
The PANC-1 cell line showed no detectable phospho- GSK-3βser9 in Western blotting analysis with a phospho-specific antibody, indicating GSK-3β activity in this cell line (Figure 4). On the other hand, phospho- GSK-3βser9 was induced after 4 mg/L gemcitabine treatment, and gradually increased with the 16 and 64 mg/L gemcitabine treatment. There was no significant difference in GSK-3β expression between the gemcitabine-treated and untreated groups.
Figure 4 Effect of gemcitabine treatment on GSK-3β expression.
DISCUSSION
Gemcitabine is a nucleotide analog that is converted to its triphosphate active form in cells and is subsequently incorporated into DNA to terminate strand elongation[13-17]. It shows a favorable clinical outcome in the treatment of pancreatic cancer and non-small cell lung cancer[14,18]. However, there have been few published in vitro analyses about gemcitabine. We therefore attempted to investigate the genes involved in the gemcitabine-induced cytotoxicity of pancreatic cancer cells. In the present study we showed that gemcitabine induced changes in expression of apoptosis-related genes in PANC-1 cells. The expression of anti-apoptotic pancreatitis-associated protein (PAP) was down-regulated, whereas the pro-apoptotic TP53INP1 and p-GSK-3β were up-regulated after being treated with gemcitabine.
Apoptosis is believed to play an important role in tumors, and both pro- and anti-apoptotic factors are simultaneously activated in tumor development and progression. In this study, we showed that PAP expression was significantly decreased in the gemcitabine-induced anti-tumor process. The growth of human pancreatic cancer may require the up-regulation of PAP expression, which would suppress the apoptosis of cancer cells[19]. PAP is one of the effectors of antiapoptosis induced by tumor necrosis factor-α through NF-κB and MAP kinases in pancreatic acinar cells[29]. It was demonstrated that the activation status of the NF-κB, Akt and MAP kinase signaling pathways is often associated with anti-apoptotic signal transduction that is linked to chemotherapeutic agents[16]. NF-κB plays an important role in oncogenesis and promotes cellular resistance to anticancer therapy. Bandala et al[30] showed that gemcitabine treatment increased the activity of NF-κB, and Alexader et al[31] showed that the induction of NF-κB by gemcitabine was dose-dependent in five pancreatic cancer cell lines. In addition, gemcitabine specifically activates p38 MAP kinases in the human pancreatic cancer cell lines, PK-1 and PCI43[16]. Taken together, these findings confirmed that PAP is involved in the gemcitabine induced-apoptosis in a dose-dependent manner.
Apoptosis is one of the major consequences of chemotherapy against malignancies. It is now established that the tumor suppressor p53 inhibits cell growth through the activation of cell cycle arrest and apoptosis. TP53INP1 is a gene that is activated by wild type p53. It cooperates with HIPK2 to promote p53 phosphorylation at ser 46, and then induces apoptosis-inducing protein activation[25,26]. In our conditions, we showed TP53INP1 was up-regulated by gemcitabine treatment from 4 mg/L to 64 mg/L, in a dose-dependent manner. TP53INP1 may be involved in gemcitabine-mediated apoptosis. The precise mechanism of the TP53INP1 increase after gemcitabine treatment is unclear. It has been shown that phosphorylation of the NH2-terminal residues of p53 mediates its stabilization and nuclear accumulation after anticancer drug treatment[28].
Beurel et al[28] has reported that GSK-3β is hyperphosphorylated on serine 9 in human hepatoma cell lines as well as in human and murine tumoral livers. We examined the impact of gemcitabine on GSK-3β activity in PANC-1 cells by measuring the phosphorylation level of GSK-3β at serine 9 as an indicator of GSK-3β inactivation. GSK-3β can act as a positive or negative physiological regulator of the p53 protein. During endoplasmic reticulum stress, p53 is inhibited through a mechanism involving its phosphorylation at ser 315 and ser 376 by GSK-3β. On the other hand, GSK-3β plays a crucial role in cell survival mediated by nuclear factor-kappaB (NF-κB) signaling. Phospho- GSK-3βser9 is an inactive form in normal tissues that suggests the regulation of the balanced expression of the active and inactive forms of this kinase. In our data, phospho- GSK-3βser9 was induced by gemcitabine treatment, indicating that GSK-3βgradually became inactive in the gemcitabine-treated cells. Our results are similar to those of Beurel et al[28], who showed that GSK-3βser9 phosphorylation by lithium treatment on tumor cells conferred resistance to anticancer therapy.
In conclusion, gemcitabine decreases cell proliferation of a human pancreatic cancer cell line, PANC-1, and promotes cellular apoptosis. The antiapoptotic gene, PAP, is down-regulated by gemcitabine treatment, whereas the pro-apoptotic TP53INP1 gene and GSK-3βser9 protein are up-regulated. Gemcitabine can induce apoptosis in cancer cells through GSK-3β and PAP inhibition, and TP53INP1 and GSK-3βser9 activation.