INTRODUCTION
Protein kinases are functionally involved in a broad variety of human cancer[1,2]. In tumor cells, it is common that key tyrosine kinases are no longer adequately controlled, and excessive phosphorylation sustains signal transduction pathways in an activated state. Receptor tyrosine kinases (RTKs) are membrane bound proteins, consisting of a ligand-binding domain at the extracellular surface, a single transmembrane segment, and a cytoplasmic part harboring the protein tyrosine kinase activity. Ligand-induced dimerization, resulting in autophosphorylation of their cytoplasmic domains, is the major mode of activation of RTKs[3]. Many oncogenic mutations in RTKs involve either point mutations leading to constitutive dimerization of the receptors or translocations causing fusion of oligomerization motifs to RTKs. Overexpression, probably caused by mutations in gene regulatory sequences or gene amplification, may lead to constitutive dimerization of RTKs. Moreover, mislocalization of RTKs due to fusion to other proteins may contribute to their oncogenic phenotype. Phosphorylated tyrosine residues in the receptor tails then function as recruitment sites for downstream signalling proteins containing phosphotyrosine-recognition domains, such as the SRC homology 2 (SH2) domain or the phosphotyrosine-binding (PTB) domain. These molecules act as relay points for a complex network of independent signalling molecules that ultimately affect gene transcription within the nucleus. c-kit (CD-117) is a transmembrane tyrosine kinase receptor in which the extracellular protein binds a ligand known as stem-cell factor (also known as Steel factor) and the intracellular portion contains the actual kinase enzymatic domain[4-6]. c-kit is similar in structure to several other RTKs with oncogenic capabilities, including platelet-derived growth factor receptor (PDGF-R) A and B, colony stimulating factor 1 receptor (CSF1-R), and fms-related tyrosine kinase 3 receptor (FLT3-R). c-kit is expressed at high levels in hematopoietic stem cells, mast cells, melanocytic cells, germ cells, and the interstitial cells of Cajal (ICC)[7-10]. The receptor forms homodimers upon ligand binding leading to receptor activation. This triggers activation of critical downstream signalling pathways, such as Ras/Raf/mitogen-activated protein kinase kinase (MAPKK)/mitogen-activated protein kinase (MAPK) (cell proliferation) and phosphatidylinositol 3-kinase (PI3K)/AKT (cell survival)[11,12]. There are receptor activating oncogenic KIT mutations which involve the extracellular (exon 9), juxtamembrane (exon 11) and kinase domains (exons 13 and 17). As a consequence, in hematologic neoplasms, the exact signalling pathways activated by the mutant KIT differ from those activated by normal KIT[13,14].
The clinical development of targeted tyrosine kinase inhibitors represents a breakthrough for cancer treatment. They are designed to take advantage of the molecular differences specific to tumor cells compared with normal tissues. The goal is to achieve tumor responses with better safety profiles than those associated with cytotoxic chemotherapies that exhibit narrow therapeutic indices and little selectivity for cancer cells over normal proliferating cells. Imatinib mesilate (STI-571, GleevecTM, Novartis), a 2-phenylaminopyrimidine, was primarily designed to treat chronic myeloid leukemia (CML) by inhibiting bcr-abl fusion protein, created by the t(9; 22) chromosomal translocation, which generates the distinctive Philadelphia (Ph) chromosome[15]. However, imatinib mesilate is not a specific bcr-abl inhibitor, its action extends to c-abl receptor, PDGF-R, and c-kit-R[15]. Moreover, it promotes NK cell activation[16] and downregulates telomerase activity[17]. Therefore, imatinib mesilate has been evaluated for the therapy of gastrointestinal stromal tumors (GISTs), because most of them characteristically show c-kit over-expression. For metastatic GISTs, imatinib mesilate is nowadays the therapeutical standard, based on a phase II study, including 147 patients with unresectable GISTs, which reported a partial response rate of 54 % and a rate of stable disease of 28 %[18]. A closer inspection of the data clearly displayed that KIT mutational status correlated with response to imatinib mesilate. Thus patients with activating KIT mutations in exon 11 had a partial response of close to 80%. In contrast, patients whose tumors expressed wild-type KIT, with no mutations, had a response rate of only 18%[19]. However, there are only a few published phase I and II studies investigating the effect of imatinib mesilate on gastrointestinal tumors, others than GISTs. Whereas three studies detected a modest level of biological effect of imatinib in hepatocellular carcinoma (HCC) and carcinoid tumor, there was no effect for the treatment of pancreatic carcinoma in two other studies[20-24]. In this study we investigated c-kit protein expression in biliary tract cancer cell lines and histological sections from 19 patients with extrahepatic cholangiocarcinoma (CC) and evaluated the efficacy of in vitro and in vivo treatment with imatinib mesilate.
MATERIALS AND METHODS
Materials
Human gallbladder cancer cell line Mz-ChA-2[25], human extrahepatic cholangiocarcinoma cell line EGI-1[26], and colorectal carcinoma cell line HT-29[27] were cultured as mono-layers in Dulbecco`s modified Eagle`s medium (DMEM, Invitrogen GmbH Karlsruhe, Germany) supplemented with 100 mL/L fetal bovine serum (FBS, Invitrogen GmbH Karlsruhe, Germany), 100 ku penicillin and 100 g/L streptomycin in humidified atmosphere of
900 mL/L air and 100 mL/L CO2 at 37 °C. Small cell lung cancer (SCLC) cell line NCI-H69[28] was kept in RPMI 1640 medium (Invitrogen GmbH Karlsruhe, Germany) containing 100 mL/L FBS and antibiotics in humidified atmosphere of 950mL/L air and 50mL/L CO2 at 37 °C. The 2-phenylaminopyrimidine derivative STI-571 (imatinib mesilate, GleevecTM) was provided by Novartis Pharma AG (Basel, Switzerland). Stock solution (10 g/L) was prepared in normal saline (NS) and stored at -20 °C. Hoechst dye was purchased from Sigma (Sigma-Aldrich Chemie GmbH Munich, Germany), rabbit polyclonal c-kit antibody (CD117 Ab-6) from Neomarkers (Dunn Labortechnik GmbH, Asbach, Germany), and CyTm3-conjugated donkey anti-rabbit antibody from Jackson ImmunoResearch (Jackson ImmunoResearch Europe Ltd., Cambridgeshire, United Kingdom). Six to eight-week-old female athymic NMRI nude mice were supplied from Taconic (Taconic Europe, Ry, Denmark) and held under pathogen-free conditions. Human care was administered, and study protocols complied with the institutional guidelines.
Immunoblotting
Cell culture mono-layers were washed twice with ice-cold PBS and lysed with buffer containing Tris-HCl (20 mmol/L, pH 7.5), NP-40 (10 g/L), Triton-X (5 g/L), NaCl (250 mmol/L), EDTA (1 mmol/L), 100 ml/L glycerol, and one tablet of complete mini-EDTA-free protease inhibitor cocktail (Boehringer, Mannheim, Germany) (in 10 mL buffer). Protein concentration was determined by the Bradford protein assay (Bio-Rad, Munich, Germany). 30 μg of cell lysates were separated on SDS-polyacrylamide gels and electroblotted onto polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Freiburg, Germany). Membranes were then incubated in blocking solution (50 g/L BSA in 10 mmol/L Tris-HCl, 140 mmol/L NaCl, 1 g/L Tween-20) (TBS-T), followed by incubation with the primary antibody at 4 °C overnight. The membranes were then washed in TBS-T and incubated with HRPO-conjugated secondary antibodies for 1 h at room temperature. Antibody detection was performed with an enhanced chemoluminescence reaction.
Immunocytochemistry
For immunocytochemistry 1 x 105 tumor cells were seeded on coverslips of 12-well plates and incubated for one day. Then, medium was removed, cells were washed with PBS and fixed with 8 g/L formaldehyde in PBS for 2 h, washed again, and then permeabilized in 3 g/L Triton-X and
10 g/L DMSO in PBS for another 10 min. After nonspecific antibody binding was blocked by incubation with
50 ml/L donkey serum in 3 g/L Triton-X and 10 g/L DMSO in PBS at room temperature for 2 h, the primary antibody was added, followed by an incubation at 4 °C overnight. The omission of primary antibody served as negative control. The next day, cells were washed again and probed with CyTm3-conjugated donkey anti-rabbit antibody at room temperature for 2 h. Then, extensive washing and nuclear staining was performed using Hoechst dye according to manufacturers instructions. Finally, coverslips were mounted on slides with 900 ml/L glycerol and slides were analyzed with a Zeiss LSM 510 laser scanning microscope (Carl Zeiss Jena GmbH, Jena, Germany).
Immunohistochemical staining
Paraffin-embedded tissue sections from resection specimens of 19 patients with extrahepatic CC were de-waxed, hydrated through graded alcohol, and immunostained with antibodies to c-kit using the avidin-biotin horseradish peroxidase method as previously described[29]. Immunoreactivity was revealed using True BlueTM peroxidase substrate (KPL, Gaithersburg, USA). The sections were counterstained with hematoxilin and mounted with Permount (Fisher, Pittsburg, USA). For the tumor tissues, the percentage of positive cells was estimated and the staining intensity was semiquantitatively recorded as 1+, 2+, or 3+. For statistical analyses, the staining results were categorized into four groups according to Went et al[30]. Tumors without any staining were considered negative. Tumors with 1+ staining intensity in less than 60% of cells and 2+ intensity in less than 30% of cells were considered weakly positive. Tumors with 1+ staining intensity in ≥ 60% of cells, 2+ intensity in 30% to 79%, or 3+ intensity in less than 30% were considered moderately positive. Tumors with 2+ intensity in ≥ 80% or 3+ intensity in ≥ 30% of cells were considered strongly positive. Only membranous or membranous plus cytoplasmic staining was considered for analysis because cytoplasmic staining alone proved to be false-positive in all preabsorption control experiments.
Inhibition of cell growth
The effect of imatinib treatment on cellular proliferation was assessed by automated cell counting (Schaerfe Casy 2.0 Cell Counter, Reutlingen, Germany) according to the manufacturers instructions. Briefly, 2 × 105 cells were seeded in T-25 cell culture flasks. Twenty-four hours after incubation, cells were treated with imatinib mesilate at 5 different concentrations (0, 1, 5, 10, and 20 µmol/L, respectively). After 6 d of incubation, cells were trypsinized, washed, and analyzed in triplicates by automated cell counting.
Animal studies
Tumors were induced by injecting 5×106 Mz-ChA-2 or EGI-1 cells in 200 µL PBS sc into the flank region of NMRI nude mice (n = 40). Treatment was started when an average tumor volume of 200 mm³ was reached (usually after 2 wk). The verum group (n = 2×10) received imatinib mesilate, dissolved in normal saline (NS), ip once a day at 50 mg/kg, whereas the control group (n = 2×10) received NS only. Treatment was continued for 14 consecutive days, tumors were daily measured with a Vernier caliper and tumor volumes were calculated using the formula tumor volume = 0.5 ×L×W², where L represents the length and W the width of the tumor. When treatment was finished, animals were sacrificed with tumors excised and weighed.
Statistical analysis
Statistical calculations were performed using SPSS, version 10.0 (SPSS Inc., Chicago, USA). Numeric data were presented as mean value with standard deviation (SD). Inter-group comparisons were performed with the Student t test. P< 0.05 was considered significant.
RESULTS
c-kit protein expression in biliary tract cancer cell lines
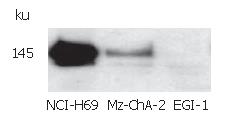
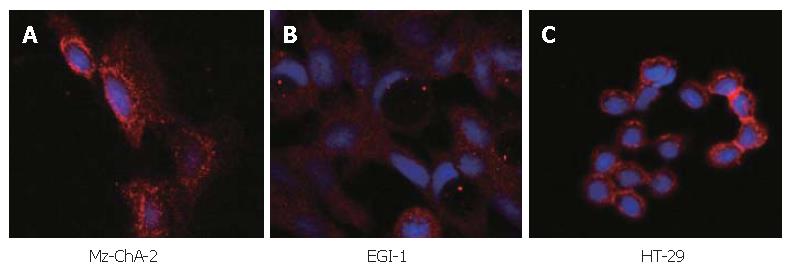
The expression of c-kit in both human biliary tract cancer cell lines Mz-ChA-2 and EGI-1 was assessed by immunoblotting. Cell lysate of SCLC cell line NCI-H69, which has been reported previously to express c-kit[28], served as positive control and showed an intense band at about 145 kDa, corresponding to c-kit receptor (Figure 1). In accordance with our previous study of c-kit m-RNA expression[31], immunoblotting of Mz-ChA-2 cell lysate demonstrated c-kit protein expression in this cell line as well. In contrast, c-kit expression was not detected in the m-RNA negative cell line EGI-1 (Figure 1). To further analyze c-kit expression in these cell lines, immunocytochemistry was performed. In this experiment, colorectal cancer cell line HT-29, which is known to express c-kit, served as positive control[27]. Immunocytochemical analysis showed a strong membraneous staining of the receptor protein in this cell line (Figure 2). In the tested biliary tract cancer cell lines, staining of c-kit revealed both cytoplasmatic and membraneous localization of receptor protein in Mz-ChA-2 cells and absence of c-kit in EGI-1 cells (Figure 2).
Figure 1 c-kit protein expression in biliary tract cancer cell lines.
Figure 2 Immunocytochemistry in biliary tract cancer cell lines of Mz-ChA-2 (A), EGI-1 (B) and HT-29 (C).
c-kit protein expression in human biliary tract cancer tissue
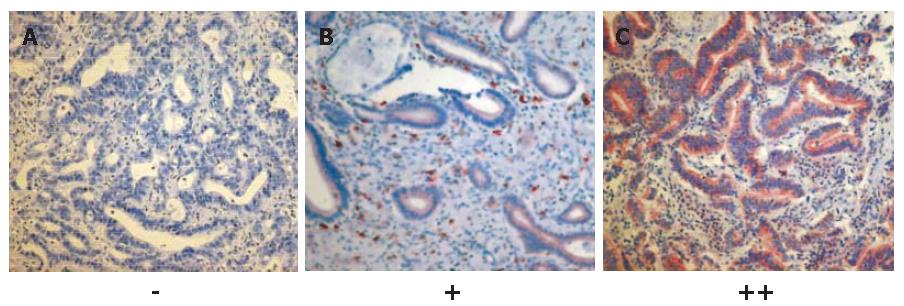
In order to analyze the expression of c-kit in human biliary tract cancer, paraffin-embedded tissue sections from 19 patients suffering from extrahepatic hilar CC were immunostained with c-kit-antibodies using the avidin-biotin horseradish peroxidase method. The clinical characteristics of the study population were as follows: median age of patients was 61 years (range, 39-74 years); UICC IA, IB, IIA, IIB, III, and IV tumor stage (UICC 2002)[32] had 1, 3, 7, 6, 1, and 1 patients, respectively; Bismuth-Corlette[33] types I, II, IIIA, IIIB, and IV were found in 1, 1, 8, 2, and 7 patients, respectively; and 16 patients had well or moderately differentiated tumors. As a result, 2 of the 19 tissues samples displayed a moderate immunostaining (++), 5 a rather weak immunostaining (+), and 12 were negative (-). Thus, 7 of 19 (37%) of the histological sections of extrahepatic hilar CC tested showed moderate to weak expression of c-kit. The immunoreactivity of c-kit was found mainly in the cell membrane (Figure 3). Highly-differentiated tumors seemed to show a higher level of c-kit expression than low-differentiated tumors.
Figure 3 c-kit protein expression in 12 (A), 5 (B) and 2 (C) human biliary tract cancer tissue samples (SABC x 200).
Inhibition of cellular growth by imatinib mesilate
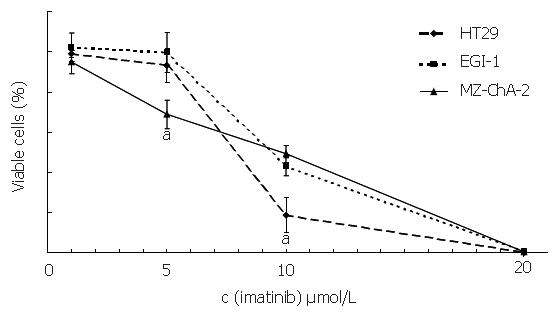
To assess the effect of imatinib mesilate treatment on cellular proliferation of both biliary tract cancer cell lines, automated cell counting was performed, using the colorectal carcinoma cell line HT-29 as a positive control[27]. After 6 d of incubation, imatinib mesilate at a low concentration of 5 µmol/L caused a significant growth inhibition in c-kit positive cell line Mz-ChA-2 (31%±7%), a moderate growth inhibition in c-kit positive cell line HT-29 (7%±9%), and no growth inhibition in c-kit negative cell line EGI-1 (0%±10%) (P < 0.05, Mz-ChA-2 vs EGI-1). Imatinib mesilate at an intermediate concentration of 10 µmol/L inhibited cellular growth of both biliary tract cancer cell lines significantly (51%±4% vs 57%±4%, Mz-ChA-2 vs EGI-1). However, this effect was even stronger for HT-29 (91%±19%) (P < 0.05, HT-29 vs Mz-ChA-2 and EGI-1). Imatinib mesilate at a higher concentration of 20 µmol/L seemed to have a general toxic effect in all cell lines (99%±1% vs 99%±1% vs 100%, Mz-ChA-2 vs EGI-1 vs HT-29) (Figure 4). The resulting IC50 values were 9.7 µmol/L, 11 µmol/L, and 9.5 µmol/L, respectively.
Figure 4 Inhibition of imatinib on cell growth (aP < 0.
05).
Inhibition of tumor cell growth by imatinib mesilate in nude mice
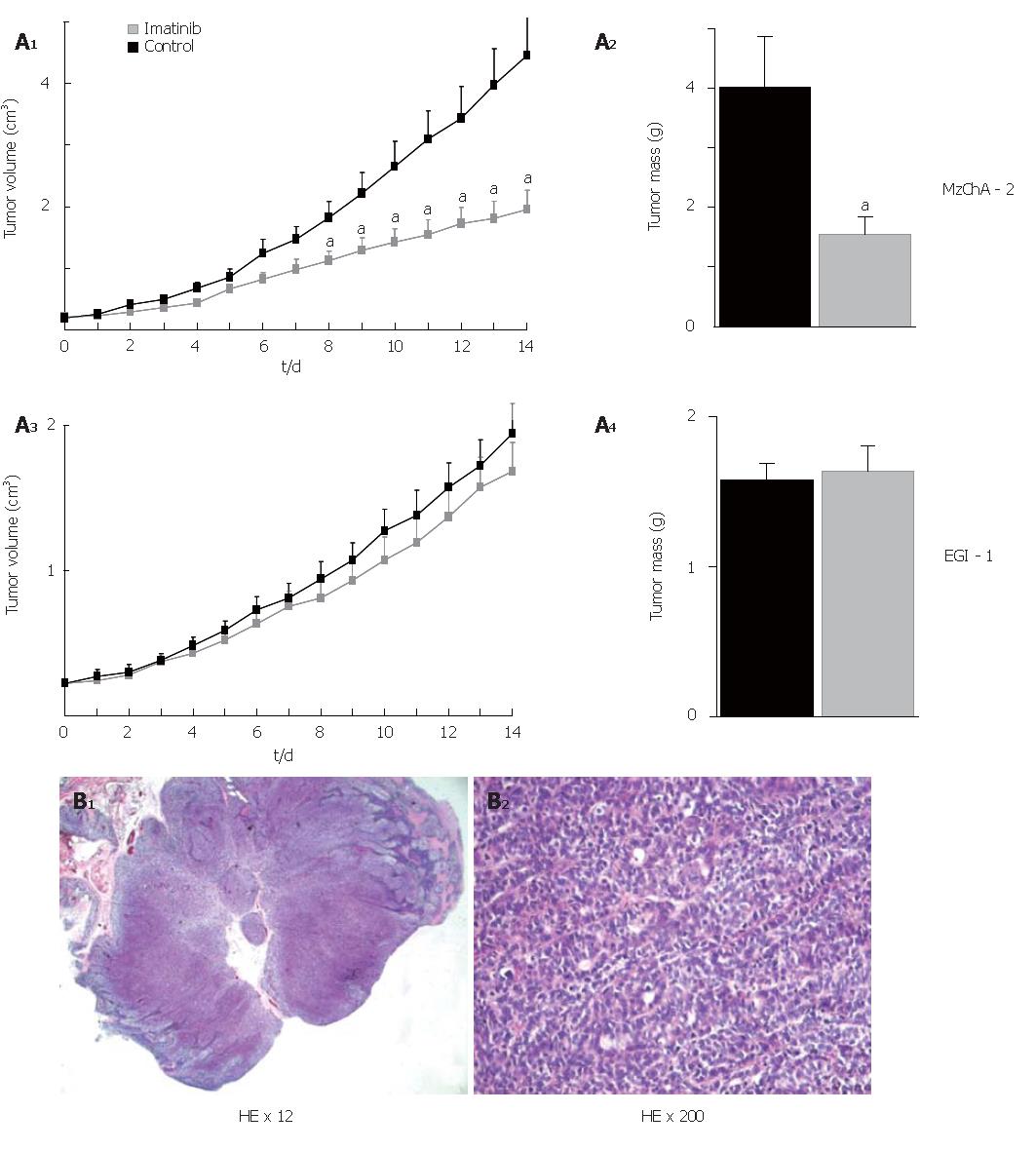
To assess the antitumor activity of imatinib mesilate in vivo, tumors were induced in nude mice by subcutaneous injection of either Mz-ChA-2 or EGI-1 cells into the flanks of the animals (n = 20 for each cell line). Treatment of mice consisted of daily ip injections of 50 mg/kg BW imatinib mesilate, and injection of NS served as control. Eight to 14 d after beginning of treatment, Mz-ChA-2 cell tumors had a significantly reduced volume in comparison to control (P < 0.05). At the end of the experiment (d 14) tumor mass was significantly diminished as compared to control (P < 0.05). In contrast to that, imatinib mesilate treatment of mice bearing EGI-1 cell tumors did not result in any change of tumor growth compared to control (Figure 5A). Hematoxilin-eosin stained paraffin-embedded tissue sections from mouse tumors showed the typical features of low-differentiated adenocarcinoma (Figure 5B).
Figure 5 In vivo treatment with imatinib mesilate in chimeric mice; A: effect on tumor volume and tumor mass (aP < 0.
05, imatinib vs control); B: hematoxilin-eosin stained paraffin-embedded tissue sections.
DISCUSSION
Non-resectable biliary tract cancer is associated with a poor prognosis due to wide resistance to chemotherapeutic agents and radiotherapy. It is therefore essential to search for new therapeutical approaches. Imatinib mesilate (STI571, GleevecTM), which was found to inhibit the bcr-abl tyrosine-kinase resulting from the translocation t(9;22) in chronic myeloid leukemia (CML), as well as c-kit and PDGF-R tyrosine kinases, may be an alternative[34]. We have previously shown expression of c-kit and PDGF-R m-RNA in 50% and 75% of 12 biliary tract cancer cell lines[31]. Activating mutations of KIT were not found, which is consistent with the data of Sihto et al[35]. This group screened 334 human cancers (32 histologic tumor types) for mutations with sensitive denaturing high-performance liquid chromatography (DHPLC) and found KIT mutations only in GIST tumors. In our study, c-kit receptor ligand SCF was present in all cells lines, respectively. We reasoned that the proliferation of these cells might be stimulated by an autocrine mechanism. In order to test this hypothesis, we treated cell lines with different concentrations of imatinib mesilate. Imatinib mesilate at an intermediate concentration of 10 µmol/L and a higher concentration of 20 µmol/L reduced survival in all cell lines examined by manual cell counting after staining with trypan blue. The reduction was statistically significant higher in c-kit positive cell lines (P < 0.02) and was independent from PDGF-R status. At 50 μmol/L, a general non-specific toxic effect occurred in all cell lines. The average IC50 for growth inhibition of c-kit positive cell lines was estimated to be 10 μmol/L. 5-Fluorouracil (5-FU) alone (0.1 µg/mL) reduced cell growth by 40 % - 50 %, while the combination with imatinib mesilate reduced cell growth by another 20% (1 µmol/L) (P = 0.008) and 30% (10 µmol/L) (P = 0.0001), respectively. Additionally, treatment of cells with imatinib mesilate ± 5-FU had no modulating effects on S-phase fraction, but was associated with a significant induction of apoptosis[31].
However, at that time it was unclear whether c-kit could be detected at the protein level and how its distribution whithin the tumor cells was. Moreover, while this work was in progress, Chiorean et al were unable to detect c-kit at protein levels in human cholangiocarcinoma cell lines KMCH-1 and Mz-CHA-1[36]. In the present study, immunoblotting confirmed the results of our previous m-RNA analysis for Mz-ChA-2 and EGI-1 cells. Immunocytochemistry with c-kit antibodies displayed a cytoplasmatic and membraneous localization of receptor protein in c-kit positive Mz-ChA-2 cells, indicating a steady receptor turnover. Cell lysates of SCLC cell line NCI-H69 and colorectal cancer cell line HT-29 served as positive controls for immunoblotting and immunocytochemistry. Both cell lines displayed a stronger protein expression that might correlate with a higher sensitivity for imatinib mesilate treatment as shown in previous studies[27,28,37,38]. In order to verify that c-kit protein is not only expressed in cholangiocarcinoma cell lines, but also in human biliary tract cancer tissue, paraffin-embedded tissue sections from 19 patients suffering from extrahepatic hilar CC were immunostained with c-kit-antibodies using avidin-biotin horseradish peroxidase method. c-kit was expressed in 7 of 19 (37%) extrahepatic human CC tissue samples, 2 showed a moderate and 5 a rather weak immunostaining. This result is comparable to a previous analysis of 13 patients with intrahepatic cholangiocarcinoma which demonstrated an expression level of 31%[39]. In contrast, a study from Argentina detected only 6% c-kit expression in 50 specimens from gallbladder carcinoma[40]. Moreover, a weak to moderate c-kit expression was shown in 7 of 12 (58%) bile duct and gallbladder adenocarcinomas by Aswad et al[41]. Finally, a recent multitumor tissue microarray (TMA) analysis by Went et al, containing more than 3500 paraffin-embedded tumor samples representing more than 120 tumor types and subtypes, and a recent TMA analysis by Sihto et al showed negative results for gallbladder carcinoma (n = 27) and cholangiocarcinoma (n = 3)[30,35]. These controversial data could be explained by different antibodies used or different tissue selections. Using automated cell counting, we tried to confirm our manual cell counting data for the c-kit positive cell line Mz-ChA-2 and the c-kit negative cell line EGI-1 after treatment with imatinib mesilate. Imatinib mesilate at a low concentration of 5 µmol/L caused a significant growth inhibition in c-kit positive cell line Mz-ChA-2 (31%), but not in c-kit negative cell line EGI-1 (0%) (P < 0.05). Imatinib mesilate at an intermediate concentration of 10 µmol/L inhibited cellular growth of both cell lines (51% vs 57%), but was most effective in colorectal carcinoma cell line HT-29 (91%). Imatinib mesilate at a higher concentration of 20 µmol/L seemed to have a general toxic effect in both cell lines. The results for these particular cell lines indicate that: (1) at a concentration of 5 µmol/L imatinib mesilate seems to exhibit a c-kit receptor dependent cell growth inhibition. The different IC50 values of 9.5 µmol/L for HT-29, 9.7 µmol/L for Mz-ChA-2, and 11 µmol/L for EGI-1 may therefore be influenced by the different level of c-kit expression; (2) at a concentration of 10 and 20 µmol/L the inhibition by imatinib mesilate seems to be no longer c-kit receptor dependent. A possible explanation may be a significant decrease in epidermal growth factor receptor (EGF-R) and focal adhesion kinase (FAK) phosphorylation at these concentrations, as observed by Chiorean et al for cholangiocarcinoma cell line KMCH-1 which was associated with a reduction in Akt activity resulting in loss of Mcl-1, a potent anti-apoptotic Bcl-2 family member[36]. Whether an additional dose dependent inhibition of telomerase activity, as observed for c-kit positive Ewing sarcoma, melanoma, myeloma, breast cancer, Fanconi anemia, and c-kit negative murine pro-B cells, plays a role in cholangiocarcinoma has not been evaluated yet[17]; (3) the assumed general toxic effect of imatinib mesilate at a concentration of 20 µmol/L indicates that even a c-kit and PDGF-R negative cell line like EGI-1 can sometimes be destroyed at this concentration whereas our previous meta-analysis of 6 c-kit m-RNA negative biliary tract cancer cell lines demonstrated a general toxic effect only at 50 µmol/L with a potential inhibition at 20 µmol/L[31].
Even more important are therefore the results from our chimeric mouse model since so far there has been no animal model published for in vivo treatment with imatinib mesilate in biliary tract cancer. At the end of a 14 d treatment period with imatinib mesilate, c-kit positive Mz-ChA-2 tumors had a significantly reduced volume and mass as compared to NS treatment (P < 0.05). In contrast to that, treatment of mice bearing c-kit negative EGI-1 tumors did not result in any change of tumor volume and mass as compared to NS treatment. These results support the notion of a c-kit receptor expression dependency of sensitivity to imatinib mesilate in vivo. At the selected dose of 50 mg/kg, which was chosen according to a study by Druker et al[42], no toxic side effects occurred. As discussed by Druker et al[43], at steady state, a once-daily administration of 400 mg imatinib mesilate, the commonly used oral dose, translates into a mean maximal serum concentration of 4.6 μmol/L. Therefore, a daily oral dose of 600 mg may be reasonable for a study with patients suffering from biliary tract cancer. Drug-related adverse effects of imatinib mesilate include nausea, vomiting, myalgias, edema, diarrhea, and, less commonly, anemia, thrombocytopenia, neutropenia, and myelosuppression[43-45]. Moreover, there are concerns regarding the drugs liver toxicity in biliary tract cancer patients since it is primarily metabolized in the liver and tumor infiltration of the liver may reduce liver function. Therefore, Eckel et al studied pharmacokinetics of imatinib mesilate in 17 patients with hepatocellular carcinoma and Child A-liver cirrhosis and compared the data with the data of 6 patients with CML and normal liver function[46]. As a result, they found no changes in pharmacokinetics in the first group of patients, thus letting assume the drugs safety in patients with hepatocellular carcinoma and Child A-liver cirrhosis. It is likely that these data can be transferred to patients with invasive biliary tract cancer. However, a phase I/II study is now required to further evaluate our preliminary in vitro and in vivo data. Therefore, our group has designed a clinical study for patients with biliary tract cancer that is now in progress.
In conclusion, c-kit expression is detectable by immunoblotting, immunocytochemistry, and immunohistochemistry at a moderate to low protein level in biliary tract cancer. Imatinib mesilate exerts marked effects on tumor growth in vitro and in vivo dependent on the level of c-kit expression. A phase I/II study has been designed to further study the role of imatinib mesilate treatment in patients with biliary tract cancer.