Published online Mar 14, 2006. doi: 10.3748/wjg.v12.i10.1551
Revised: October 1, 2005
Accepted: November 18, 2005
Published online: March 14, 2006
AIM: To study the baculovirus/mammalian cell system for efficient expression of functional large hepatitis delta antigen (L-HDAg).
METHODS: A recombinant baculovirus expressing histidine-tagged L-HDAg (L-HDAgH) was constructed to transduce baby hamster kidney (BHK) cells by a simplified transduction protocol.
RESULTS: The recombinant baculovirus transduced BHK cells with efficiencies higher than 90% as determined by flow cytometry. The expression level was significantly higher than that obtained by plasmid transfection and was further enhanced 3-fold to around 19 pg/cell by the addition of 10 mmol/L sodium butyrate. Importantly, the expressed L-HDAgH was localized to the cell nucleus and correctly isoprenylated as determined by immunofluorescence labeling and confocal microscopy. Moreover, L-HDAgH interacted with hepatitis B surface antigen to form virus-like particles.
CONCLUSION: The fusion with histidine tags as well as overexpression of L-HDAgH in the baculovirus-transduced BHK cells does not impair the biological functions. Taken together, the baculovirus/mammalian cell system offers an attractive alternative for high level expression of L-HDAgH or other proteins that require extensive post-translational modifications.
- Citation: Chiang YW, Wu JC, Wang KC, Lai CW, Chung YC, Hu YC. Efficient expression of histidine-tagged large hepatitis delta antigen in baculovirus-transduced baby hamster kidney cells. World J Gastroenterol 2006; 12(10): 1551-1557
- URL: https://www.wjgnet.com/1007-9327/full/v12/i10/1551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i10.1551
Hepatitis delta virus (HDV) is a subviral satellite and requires hepatitis B virus to supply envelope proteins (HBsAg) for its packaging, secretion and infection[1,2]. HDV superinfection of HBV carriers can lead to fulminant hepatic failure and very likely the progression to liver cirrhosis[3], consequently HDV imposes a huge threat to the health of 350 million HBV carriers worldwide. However, effective vaccines or diagnostic assays remain unavailable. The RNA genome of HDV encodes the large and small forms of hepatitis delta antigens (L-HDAg and S-HDAg). The 24 kd S-HDAg and 27 kd L-HDAg are identical in sequence except that L-HDAg contains additional 19 amino acids at the C-terminus[1,4]. The C-terminus extension of L-HDAg allows for the isoprenylation which is crucial for the interaction with S-HDAg and HBsAg for the formation of virions. It has been established that cell-mediated immunities are important in controlling HDV infection[5,6], thus an effective T-cell stimulating HDV vaccine would be highly desired. In this regard, L-HDAg may represent an excellent vaccine candidate because L-HDAg (expressed by a DNA vaccine) induces significant titers of anti-HDV antibodies as well as strong cell-mediated immune responses in mice[7]. Moreover, L-HDAg is a dominant repressor for HDV replication[8], thus implicating its application as an anti-HDV drug in addition to being a vaccine. However, efforts to characterize and evaluate the immunological properties of L-HDAg have been hampered due to the lack of a proper method for efficient expression and purification of L-HDAg.
Baculovirus (Autographa californica multiple nuclear polyhedrosis virus, AcMNPV) has been widely utilized for protein expression in insect cells. Since the finding that baculovirus is capable of transducing mammalian cells in 1995, baculovirus has been developed as a vector for in vitro and in vivo gene therapy studies and many other applications (for review, see[9,10]). Recently, the applications of baculovirus have been further expanded to the identification of MHC class I mimotopes[11] and genetic modification of primary chondrocytes[12]. Furthermore, our group has also demonstrated the feasibility of producing HDV virus-like particles in Huh-7 cells[13]. All these studies suggest that baculovirus may be an ideal tool for the expression of functional, complex eukaryotic proteins owing to the following advantages: (1) Baculovirus transduction is highly efficient for many cell types and generally causes no appreciable cytopathic effect[10,14]; (2) The large AcMNPV genome (about 130 kb) confers the virus huge cloning capacity to harbor multiple genes or large inserts[15]; (3) The construction, manipulation and production of recombinant baculovirus are easy.
Considering the need for efficient expression of L-HDAg and the advantages of baculovirus/mammalian cell system, we constructed a recombinant baculovirus encoding histidine-tagged L-HDAg (L-HDAgH) for rapid and high level expression in transduced BHK cells. The high level expression and subsequent purification may allow us to obtain sufficient quantities of functional L-HDAgH for further evaluation of its immunological properties and its potential as a vaccine candidate. The success of this study supports the notion that the baculovirus/mammalian cell system can be a platform for efficient expression of eukaryotic proteins requiring post-translational modifications.
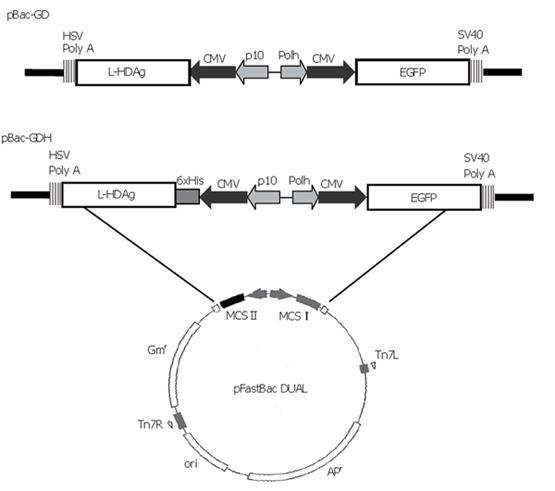
The transfer plasmid was generated using pFastBacTM DUAL (Invitrogen) as the backbone, which contained two multiple cloning sites (MCS). The gene encoding enhanced green fluorescent protein (EGFP), along with the upstream cytomegalovirus immediate-early (CMV-IE) promoter, was amplified by polymerase chain reaction (PCR) from pEGFP-C1 plasmid (Clontech) and inserted into MCS I under the polyhedrin promoter. The cDNA encoding L-HDAg was PCR-amplified from p2577-1L[16] and subcloned into pcDNA4/HisMax-TOPO (Invitrogen), thus a His6 tag was fused at the N-terminus of L-HDAg under the control of CMV-IE promoter. The whole expression cassette was PCR-amplified again and subcloned into Kpn I site of MCS II. The resultant plasmid was designated pBac-GDH (Figure 1). Another plasmid, pBac-GD, was previously constructed to harbor the genes encoding L-HDAg and EGFP[13] (Figure 1). The recombinant baculoviruses were subsequently generated using the Bac-to-Bac® system (Invitrogen) and designated as Bac-GDH and Bac-GD. Another recombinant baculovirus co-expressing HBsAg and EGFP, Bac-GB, was constructed previously[13].
BHK cells were cultured in Dulbecco’s minimal essential medium (DMEM, Sigma) supplemented with 100 mL/L heat inactivated fetal bovine serum (FBS; Gibco), 2 mmol/L L-glutamine (Gibco) and non-essential amino acids (Gibco). The cells were maintained at 37 °C in a humidified incubator containing 5 % CO2. Insect cell Sf-9 was maintained in TNM-FH medium (Sigma) supplemented with 10 mL/L FBS for baculovirus generation, amplification and titration according to standard protocols[17]. The viruses were not concentrated by ultracentrifugation. The titer of Bac-GDH was 8.3×108 plaque forming units per ml.
A simple process eliminating the need for virus ultracentrifugation[18] was adopted for transduction. Briefly, BHK cells cultured in 6-well plates (5×105 cells per well) were washed with PBS, and then transduced simply by adding 100 μL unconcentrated virus solution and
400 μL PBS. The plates (or dishes) were shaken on a rocking platform for 6 h at 25 °C. After the virus incubation, the virus solution was aspirated. The wells were replenished with 2 mL complete medium with or without sodium butyrate and incubated at 37 °C. The cells grown on 10-cm dishes were transduced similarly, except that larger volumes of virus (800 μL) and PBS (3.2 mL) were used.
BHK cells were singly transduced by Bac-GDH (or Bac-GD), or co-transduced by Bac-GDH and Bac-GB. To detect intracellular L-HDAgH (or L-HDAg), the cells were harvested at 2 dpt (days post-transduction) and resuspended in TE buffer containing phenylmethylsulfonylfluoride (PMSF). The proteins were released by sonication on ice and separated from debris by centrifugation. To detect secreted HBsAg and L-HDAgH, virus-like particles released into conditioned medium from co-transduced cells were concentrated by 200 g/L sucrose-cushioned ultracentrifugation as described previously[13]. The pellet was resuspended in TE buffer for Western blot. The intracellular or extracellular samples were resolved on 120 g/L gels and transferred onto nitrocellulose membranes. The L-HDAgH (or L-HDAg) was probed by human anti-HDV sera[19] or mouse anti-His6 mAb (Amersham Biosciences). The HBsAg was probed by anti-HBsAg mAb (A10F1, a kind gift from Prof. S.C. Lee, National Taiwan University). The secondary antibodies were AP-conjugated goat anti-human or anti-mouse IgG (Kirkegaard and Perry Laboratories). The bands were developed using BCIP/NBT reagent (Sigma). The densitometry was performed by scanning the bands on the membranes and analyzing by Scion Image Shareware (Scion Corporation). For estimation of the product yield, serially diluted purified protein was used as the standard and analyzed by scanning densitometry.
The transduced cells were harvested by trypsin/EDTA, washed and resuspended in PBS for flow cytometry (FACSCalibur, Becton Dickinson) analysis according to the manufacturer’s instruction. The percentage of cells emitting fluorescence (% GFP + cells) and mean fluorescence intensity (MFI) of each sample were measured 3 times by counting 10 000 cells in each measurement. The mean ± standard deviation (SD) of 3 independent experiments are presented as arbitrary units (au).
BHK cells were seeded onto 22 mm×22 mm glass slides and transduced in duplicate by Bac-GDH at a multiplicity of infection of 280. The cells were fixed at 24 h post-transduction (hpt) with methanol/acetone (1:1) for 5 min at -20 °C, rinsed with PBS and then blocked with PBS containing 20 g/L bovine serum albumin (BSA) for 30 min at 37 °C. For the detection of subcellular localization, L-HDAgH was probed with 150 μL anti-HDAg mAb (HP6A1, 1:300 dilution)[16] for 1 h at 37 °C and washed with PBS 3×5 min. L-HDAgH was subsequently labeled using Cy3-conjugated goat anti-mouse IgG (1:25 dilution, Sigma). For simultaneous nuclear staining, SYBR green I (1: 40 000 dilution, BioWhittaker) was added together with the secondary antibody. For isoprenylation analysis, L-HDAgH was first probed with primary antibody solution containing HP6A1 and anti-farnesyl mAb (Sigma) for 1 h at 37 °C, followed by the labeling with secondary antibody solution containing FITC-conjugated anti-mouse and TRITC-conjugated anti-rabbit mAb (Jackson ImmunoResearch) for 1 h at 37 °C. The slides were washed with PBS 3×5 min, mounted using glycerol/PBS (3:1) mounting solution and observed by a confocal microscope (TCS SP2, Leica, Germany).
The His6-tagged L-HDAg was purified by immobilized metal affinity chromatography (IMAC) under denaturing conditions. The cells were resuspended in 10 ml binding buffer A (20 mmol/L Tris-HCl, 500 mmol/L NaCl,
8 mol/L urea and 10 mL/L PMSF, pH 7.8), sonicated for 5 min and centrifuged (12000 r/min for 30 min). After centrifugation, the supernatant was loaded cyclically for 1 h at room temperature to the Fast-flow Protein Liquid Chromatography (ÄKTA FPLC, Amersham Biosciences) system in which the column contained 5 mL Ni2+-coupled Chelating Sepharose Fast Flow resin. Bound proteins were eluted by a mixture of elution buffers A and B (20 mmol/L Tris-HCl, 500 mmol/L NaCl, 500 mmol/L imidazole, pH 7.8) at 1 mL/min at 4 °C. The FPLC system generated step gradients by varying the volume ratios of buffers A and B so that the urea concentration decreased while the imidazole concentration increased stepwise. The eluate was collected in 1.5 mL fractions and then analyzed by Western blots and SDS-PAGE.
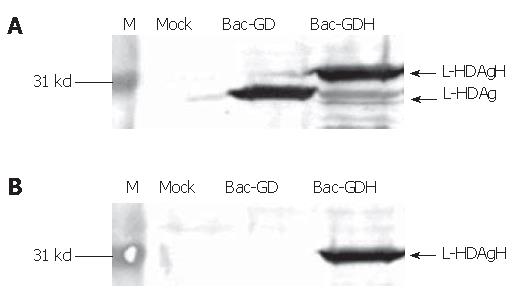
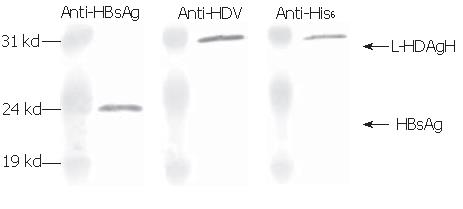
The recombinant baculoviruses designed to express L-HDAg with or without His6 tags were constructed and designated Bac-GDH and Bac-GD (Figure 1). To confirm the correct expression of L-HDAgH in BHK cells, the cells grown on 10-cm dishes were transduced by Bac-GD or Bac-GDH, harvested at 2 dpt and the cell lysates were analyzed by Western blot. Figure 2A depicts that the mock-transduced cells expressed no protein recognized by the anti-HDV serum, while the Bac-GD-transduced cells expressed a protein whose molecular mass (27 kd) corresponded to that of authentic L-HDAg, indicating the specificity of the serum. Similarly, the Bac-GDH-transduced cells expressed a protein recognized by the antibody, but with a slightly higher molecular mass (about 31 kd), thus suggesting the fusion of the His6 tag to L-HDAg. The identities of the proteins were further confirmed by analysis of the lysates using anti-His6 mAb (Figure 2B). As expected, no His6 tagged proteins were found in the mock- or Bac-GD-transduced cell lysates. In contrast, the 31 kd protein expressed by Bac-GDH was clearly visible. Figures 2A, B collectively confirme the expression of His6 tagged L-HDAg in the BHK cells.
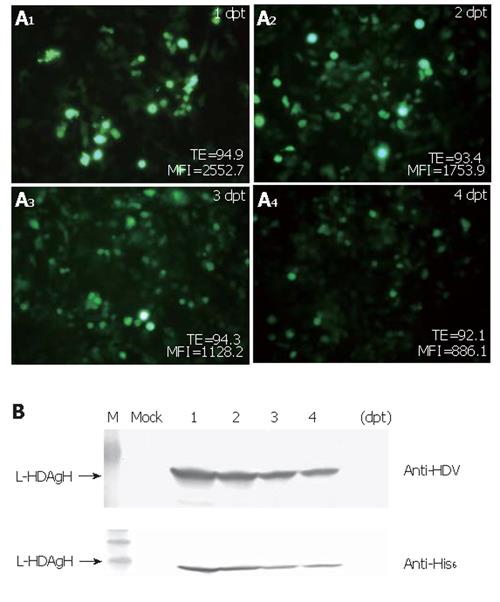
To determine how efficiently the virus could transduce BHK cells and how the protein accumulation varied over time post-transduction, BHK cells were transduced and photographed using the fluorescence microscope. The percentages of GFP+ cells and the mean fluorescence intensities (MFI) were monitored over time by flow cytometry. As shown in Figure 3A, the percentage of GFP+ cells was up to 95 % at 1 dpt and remained higher than 90 % for 4 d, but the mean fluorescence intensities declined over time. Figure 3B shows the time-course profile of L-HDAgH expression and depicts that the expression reached the maximum rapidly at 1-2 dpt. The protein concentration declined slightly thereafter, but remained high at 4 dpt.
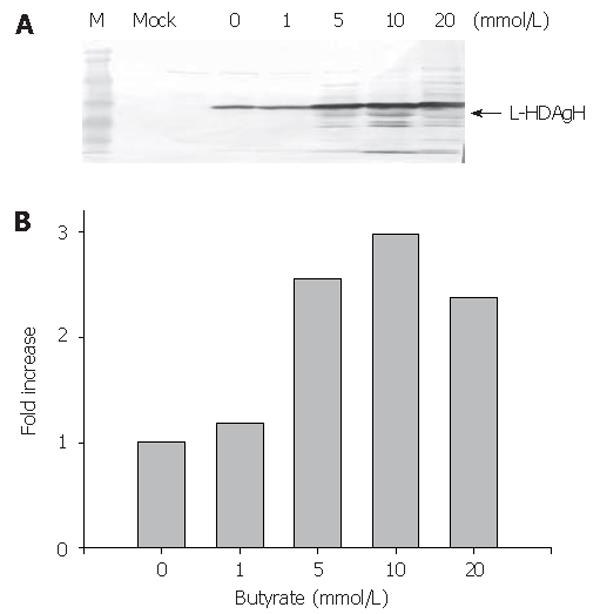
To enhance the L-HDAgH expression, the cells were transduced on 10-cm dishes as described above, but varying concentrations of sodium butyrate, a histone deacetylase inhibitor shown to loosen the condensed chromosome and up-regulate the protein expression in mammalian cell system[20,21], were supplemented to the medium after transduction. The cells were harvested at 2 dpt and the lysates were analyzed by Western blot. Figure 4A depicts a clear dependence of the L-HDAgH expression on the sodium butyrate concentrations. The scanning densitometry quantitatively revealed an about 3-fold enhancement in L-HDAgH expression by 10 mmol/L sodium butyrate, compared to the control without sodium butyrate (Figure 4B). The highest protein yield was roughly estimated to be about 19 pg/cell by scanning densitometry using serially diluted purified protein as the standard. At a higher concentration (i.e. 20 mmoL/L), the overall L-HDAgH expression dropped slightly probably due to high cytotoxicity associated with sodium butyrate[22].
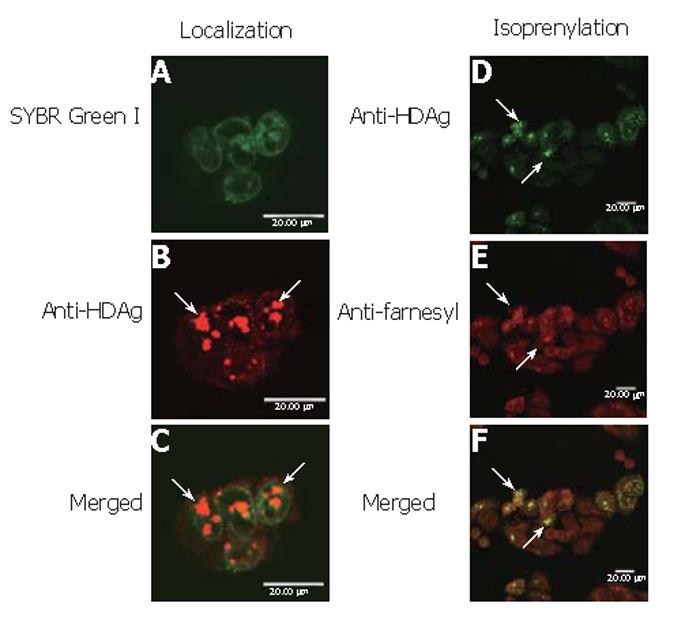
To determine whether baculovirus transduction and protein overexpression affected the cell physiology and deterred the correct protein localization, BHK cells were transduced by Bac-GDH, labeled by SYBR Green I and anti-HDAg mAb and examined by confocal microscopy. As shown, the nuclei were clearly marked by SYBR Green I (Figure 5A) while L-HDAgH was marked by clusters of red dots (Figure 5B). The merged photograph (Figure 5C) clearly illustrates the co-localization of L-HDAgH with the nuclei, confirming that the expressed L-HDAgH was localized within the nuclei.
To evaluate whether L-HDAgH was properly isoprenylated, Bac-GDH-transduced BHK cells were subject to immunofluorescence double labeling using anti-HDAg and anti-farnesyl mAb. Figure 5D shows the green dots marking the presence of L-HDAgH using anti-HDAg mAb, while Figure 5E illustrates the detection of isoprenylation using anti-farnesyl mAb as evidenced by the red dots. The merged photograph (Figure 5F) clearly illustrates the co-localization of both red and green dots, indicating that the baculovirus-expressed L-HDAgH was isoprenylated.
Since the assembly of HDV virions or virus-like particles (VLP) is an important function of L-HDAg, whether L-HDAgH remained capable of assembling into VLP was investigated. It has been shown that L-HDAg along with HBsAg was necessary and sufficient for the assembly of HDV VLP[13], thus BHK cells were co-transduced by Bac-GB and Bac-GDH. The conditioned medium was collected, concentrated and analyzed by Western blot. Figure 6 shows that the pellet contained proteins recognized by anti-HBsAg, anti-HDAg and anti-His6 antibodies, confirming the simultaneous secretion of both HBsAg and L-HDAgH into the medium. Because L-HDAgH alone was localized within the nucleus, the co-secretion proved the assembly and secretion of HDV VLP[23-25].
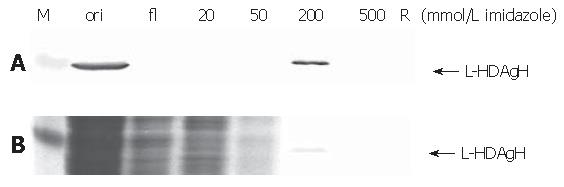
Thanks to the fused His6 tag, L-HDAgH was purified by IMAC. Under the native conditions, however, the protein binding to the resin was very weak (not shown). Therefore, the protein was denatured by 8 mol/L urea and purified under denaturing conditions. The Western blot (upper panel, Figure 7) shows that the flow through contained no L-HDAgH, indicating significantly improved binding. The proteins were then eluted with a mixture of buffers A and B with decreasing urea concentration and increasing imidazole concentration. The L-HDAgH was eluted by 200 mmol/L imidazole buffer (4.8 mmol/L urea). The purity was 7 % as revealed by SDS-PAGE (lower panel, Figure 7).
As mentioned above, L-HDAg may represent an excellent vaccine candidate, but characterization and evaluation of the immunological properties of L-HDAg have been impeded due to the lack of a proper method for efficient expression and purification. Although attempts to express L-HDAg using E coli or baculovirus/insect cell systems have been made, both systems are not suited for L-HDAg expression because the former lacks post-translational modification while the latter results in rapid degradation of L-HDAg after 2 d post-infection[26]. Besides, L-HDAg expressed in insect cells induces cell cycle arrest[27]. The integration of L-HDAg gene into the chromosome of mammalian cells for stable expression may offer another option, but has not been reported probably because L-HDAg is a nuclear protein, the accumulation of which can result in significant cytotoxicity. Transient expression in mammalian cells mediated by other virus systems (e.g. vaccinia virus) may be possible as well, but these viruses lead to cell death and lysis, thus potentially undermining the post-translational modification at later stage. Owing to these restrictions, L-HDAg expression is mainly achieved by transfecting plasmids expressing L-HDAg into hepatoma cells (e.g. Huh-7 and HepG2). The plasmid transfection into hepatocytes, however, is notorious for its low efficiency and low expression level, consequently, highly sensitive detection methods such as isotopic labeling and chemiluminescence enhancement are required to detect the expression.
To express functional L-HDAg efficiently and rapidly for research purposes, the baculovirus/mammalian cell system was employed. Using PBS as the surrounding solution, the efficiency of baculovirus-mediated gene delivery into BHK cells was up to 94 % (Figure 3A), which far exceeded 20 %-30 % normally achieved by plasmid transfection. The detection of L-HDAgH using anti-HDV sera and anti-His6 mAb (Figure 2) confirmed that the expressed L-HDAgH possessed the fused His6 tag and was similar in size and antigenic properties to those of authentic proteins. The expression level was apparently higher than that can be obtained using plasmid transfection, as evidenced by the easy detection using BCIP/NBT as the color developing reagent, which was significantly less sensitive than chemiluminescence enhancement. Under the transcriptional control of CMV-IE promoter, the L-HDAgH expression peaked at day 1-2 and gradually declined thereafter, which probably stemmed from cell proliferation and attenuation of promoter activity. Albeit this, the expression level could be up-regulated by sodium butyrate and reached the maximum (at about 19 pg/cell) at 10 mmol/L sodium butyrate (Figure 4A).
Besides the high transduction efficiency and high expression level, Figures 5A-C illustrate that L-HDAgH formed speckles localized within the nuclei of BHK cells, which is the subcellular location of authentic L-HDAg[28]. The formation of nuclear speckles is a sign of isoprenylation[29] and could have stemmed from 1) the formation of dimmers or multimers of L-HDAg between the coiled-coiled domains or 2) the interaction of L-HDAg with host proteins such as nucleolin and delta-interacting protein A[30]. The formation of aggregates has been previously observed for L-HDAg expressed in hepatoma cell lines via plasmid transfection[28], and explains the low binding of L-HDAgH to Ni2+-coupled resin under native conditions and the low purity in subsequent purification steps. Further improvement in the purification process is required and is currently underway. Furthermore, Figures 5D-5F revealed that L-HDAgH underwent proper isoprenylation, which is a prerequisite for the interaction of L-HDAg with HBsAg (and hence the virion formation). The Western blot analysis of concentrated pellets (Figure 6) further confirmed the assembly and secretion of HDV VLP. The retention of isoprenylation as well as formation of VLP implied that the biological function of L-HDAgH was not impaired by the His6 tag[31,32].
In summary, our data solidly confirm that the overexpression of His6 tagged L-HDAgH in baculovirus-transduced mammalian cells does not hamper the proper localization and processing. The data also suggest the maintenance of accurate structure and appropriate biological functions and hence validate the use of baculovirus/mammalian cell system for the production of eukaryotic proteins requiring extensive post-translational modifications. This is partly attributed to the fact that baculovirus does not cause cell lysis or induce cytotoxicity. Additionally, the baculovirus system enables protein production in BHK cells which is one of the few cell lines approved for biopharmaceutical production. Moreover, the transduction protocol eliminates the need for virus concentration by ultracentrifugation so that transduction can be carried out in the bioreactor simply by incubating the virus solution/PBS with the cells, which otherwise is difficult for plasmid transfection. Since process scale-up of BHK culture has been well established, large scale production of L-HDAg can be made possible simply by transferring this process to a bioreactor (e.g. a packed bed bioreactor).
The authors thank Professor WJ Syu for providing antibody HP6A1, Professor Shin-Wen Sung and Dr. Sung-Chin Chen for technical assistance in confocal microscopy.
S- Editor Pan BR L- Editor Zhu LH E- Editor Bai SH
| 1. | Bonino F, Heermann KH, Rizzetto M, Gerlich WH. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol. 1986;58:945-950. [PubMed] |
| 2. | Rizzetto M, Canese MG, Aricò S, Crivelli O, Trepo C, Bonino F, Verme G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 639] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 3. | Rizzetto M, Canese MG, Gerin JL, London WT, Sly DL, Purcell RH. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J Infect Dis. 1980;141:590-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 343] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Bergmann KF, Gerin JL. Antigens of hepatitis delta virus in the liver and serum of humans and animals. J Infect Dis. 1986;154:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Karayiannis P, Goldin R, Luther S, Carman WF, Monjardino J, Thomas HC. Effect of cyclosporin-A in woodchucks with chronic hepatitis delta virus infection. J Med Virol. 1992;36:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Nisini R, Paroli M, Accapezzato D, Bonino F, Rosina F, Santantonio T, Sallusto F, Amoroso A, Houghton M, Barnaba V. Human CD4+ T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profiles. J Virol. 1997;71:2241-2251. [PubMed] |
| 7. | Huang YH, Wu JC, Tao MH, Syu WJ, Hsu SC, Chi WK, Chang FY, Lee SD. DNA-Based immunization produces Th1 immune responses to hepatitis delta virus in a mouse model. Hepatology. 2000;32:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kuo MY, Chao M, Taylor J. Initiation of replication of the human hepatitis delta virus genome from cloned DNA: role of delta antigen. J Virol. 1989;63:1945-1950. [PubMed] |
| 9. | Hu YC. Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacol Sin. 2005;26:405-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Kost TA, Condreay JP. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 2002;20:173-180. [RCA] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Rubtsov A, Heiser R, White J, Crawford F, Marrack P, Kappler JW. Using a baculovirus display library to identify MHC class I mimotopes. Proc Natl Acad Sci U S A. 2005;102:2476-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Ho YC, Chen HC, Wang KC, Hu YC. Highly efficient baculovirus-mediated gene transfer into rat chondrocytes. Biotechnol Bioeng. 2004;88:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Wang KC, Wu JC, Chung YC, Ho YC, Chang MD, Hu YC. Baculovirus as a highly efficient gene delivery vector for the expression of hepatitis delta virus antigens in mammalian cells. Biotechnol Bioeng. 2005;89:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Sandig V, Hofmann C, Steinert S, Jennings G, Schlag P, Strauss M. Gene transfer into hepatocytes and human liver tissue by baculovirus vectors. Hum Gene Ther. 1996;7:1937-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Cheshenko N, Krougliak N, Eisensmith RC, Krougliak VA. A novel system for the production of fully deleted adenovirus vectors that does not require helper adenovirus. Gene Ther. 2001;8:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Hsu SC, Lin HP, Wu JC, Ko KL, Sheen IJ, Yan BS, Chou CK, Syu WJ. Characterization of a strain-specific monoclonal antibody to hepatitis delta virus antigen. J Virol Methods. 2000;87:53-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | O'Reilly D, Miller L, Luckow V. Baculovirus expression vectors: a laboratory manual. New York: W.H. Freeman and Co. 1992;109-215. |
| 18. | Hsu CS, Ho YC, Wang KC, Hu YC. Investigation of optimal transduction conditions for baculovirus-mediated gene delivery into mammalian cells. Biotechnol Bioeng. 2004;88:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Hsu SC, Syu WJ, Ting LT, Wu JC. Immunohistochemical differentiation of hepatitis D virus genotypes. Hepatology. 2000;32:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Hu YC, Tsai CT, Chang YJ, Huang JH. Enhancement and prolongation of baculovirus-mediated expression in mammalian cells: focuses on strategic infection and feeding. Biotechnol Prog. 2003;19:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Mimura Y, Lund J, Church S, Dong S, Li J, Goodall M, Jefferis R. Butyrate increases production of human chimeric IgG in CHO-K1 cells whilst maintaining function and glycoform profile. J Immunol Methods. 2001;247:205-216. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Kim YB, Ki SW, Yoshida M, Horinouchi S. Mechanism of cell cycle arrest caused by histone deacetylase inhibitors in human carcinoma cells. J Antibiot (Tokyo). 2000;53:1191-1200. [RCA] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Sheu SY, Chen KL, Lee YW, Lo SJ. No intermolecular interaction between the large hepatitis delta antigens is required for the secretion with hepatitis B surface antigen: a model of empty HDV particle. Virology. 1996;218:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Wang CJ, Sung SY, Chen DS, Chen PJ. N-linked glycosylation of hepatitis B surface antigens is involved but not essential in the assembly of hepatitis delta virus. Virology. 1996;220:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Wang CJ, Chen PJ, Wu JC, Patel D, Chen DS. Small-form hepatitis B surface antigen is sufficient to help in the assembly of hepatitis delta virus-like particles. J Virol. 1991;65:6630-6636. [PubMed] |
| 26. | Hwang SB, Lee CZ, Lai MM. Hepatitis delta antigen expressed by recombinant baculoviruses: comparison of biochemical properties and post-translational modifications between the large and small forms. Virology. 1992;190:413-422. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Hwang SB, Park KJ. Cell cycle arrest mediated by hepatitis delta antigen. FEBS Lett. 1999;449:41-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Wu JC, Chen CL, Lee SD, Sheen IJ, Ting LP. Expression and localization of the small and large delta antigens during the replication cycle of hepatitis D virus. Hepatology. 1992;16:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Shih KN, Lo SJ. The HDV large-delta antigen fused with GFP remains functional and provides for studying its dynamic distribution. Virology. 2001;285:138-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Moraleda G, Dingle K, Biswas P, Chang J, Zuccola H, Hogle J, Taylor J. Interactions between hepatitis delta virus proteins. J Virol. 2000;74:5509-5515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Lee CZ, Chen PJ, Lai MM, Chen DS. Isoprenylation of large hepatitis delta antigen is necessary but not sufficient for hepatitis delta virus assembly. Virology. 1994;199:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |