Published online Jan 7, 2006. doi: 10.3748/wjg.v12.i1.60
Revised: June 8, 2005
Accepted: June 9, 2005
Published online: January 7, 2006
AIM: To examine whether antithrombin (AT) could prevent hepatic ischemia/reperfusion (I/R)-induced hepatic metastasis by inhibiting tumor necrosis factor (TNF)-α-induced expression of E-selectin in rats.
METHODS: Hepatic I/R was induced in rats and mice by clamping the left branches of the portal vein and the hepatic artery. Cancer cells were injected intrasplenically. The number of metastatic nodules was counted on day 7 after I/R. TNF-α and E-selectin mRNA in hepatic tissue, serum fibrinogen degradation products and hepatic tissue levels of 6-keto-PGF1α, a stable metabolite of PGI2, were measured.
RESULTS: AT inhibited increases in hepatic metastasis of tumor cells and hepatic tissue mRNA levels of TNF-α and E-selectin in animals subjected to hepatic I/R. Argatroban, a thrombin inhibitor, did not suppress any of these changes. Both AT and argatroban inhibited I/R-induced coagulation abnormalities. I/R-induced increases of hepatic tissue levels of 6-keto-PGF1α were significantly enhanced by AT. Pretreatment with indomethacin completely reversed the effects of AT. Administration of OP-2507, a stable PGI2 analog, showed effects similar to those of AT in this model. Hepatic metastasis in congenital AT-deficient mice subjected to hepatic I/R was significantly increased compared to that observed in wild-type mice. Administration of AT significantly reduced the number of hepatic metastases in congenital AT-deficient mice.
CONCLUSION: AT might reduce I/R-induced hepatic metastasis of colon cancer cells by inhibiting TNF-α-induced expression of E-selectin through an increase in the endothelial production of PGI2. These findings also raise the possibility that AT might prevent hepatic metastasis of tumor cells if administered during the resection of liver tumors.
- Citation: Kurata M, Okajima K, Kawamoto T, Uchiba M, Ohkohchi N. Antithrombin reduces reperfusion-induced hepatic metastasis of colon cancer cells. World J Gastroenterol 2006; 12(1): 60-65
- URL: https://www.wjgnet.com/1007-9327/full/v12/i1/60.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i1.60
Hepatic ischemia/reperfusion (I/R) is sometimes induced by temporary clamping of the portal triad during resection of a liver tumor and a portal vein for advanced pancreatic head cancer[1,2]. Hepatic I/R may promote hematogenous liver metastases of cancer cells that detach during the surgical procedure[3]. Endothelial leukocyte adhesion molecules such as E-selectin and intercellular adhesion molecule-1 have been demonstrated to be critically involved in the adhesion of tumor cells to endothelial cells, thereby promoting the metastasis of tumor cells[4-8]. Expression of these adhesion molecules in endothelial cells is markedly enhanced by pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, which plays a critical role in the development of I/R-induced liver injury[9,10].
Antithrombin (AT), a physiological serine protease inhibitor, plays a critical role in the regulation of the coagulation cascade[11]. In addition to such regulatory role in the coagulation system, AT exerts an anti-inflammatory activity by increasing the endothelial production of prostacyclin (PGI2), which is capable of inhibiting TNF-α production by monocytes[12-14]. According to this anti-inflammatory property, AT reduces I/R-induced liver injury by inhibiting TNF-α production in rats[15,16]. These observations suggest that AT might reduce I/R-induced hepatic metastasis of cancer cells by decreasing the expression of leukocyte adhesion molecules such as E-selectin through an increase in the endothelial production of PGI2. In the present study, we have examined whether AT could reduce the metastasis of cancer cells injected intrasplenically in rats and congenital AT-deficient mice subjected to hepatic I/R.
AT and argatroban ((2R,4R)4-methyl-{N2-[(3-methyl-1,2,3,4, tetrahydro-8-quinolyl) sulfonyl]-L-arginyl}-2-piperidinecarboxylic acid monohydrate) were obtained from the Mitsubishi-Welpharma Co., Ltd. (Osaka, Japan). OP-2507 (15 cis-(4-n-propylcyclohexyl)-16,17,18,19,20-pentanor-9-deoxy-6,9-α-nitrilo-prostaglandin F1 methyl ester), a prostacyclin analog, was obtained from the Ono Pharmaceutical Co., Ltd. (Osaka, Japan). Indomethacin (IM), xylazine and RPMI 1640 were purchased from Sigma (St. Louis, MO, USA). Penicillin G, streptomycin, and fetal bovine serum (FBS) were obtained from Invitrogen (Gaithersburg, MD, USA). Ketamine hydrochloride was purchased from Parke-Davis (Morris Plains, NJ, USA). All other reagents were of analytical grade.
Pathogen-free male Fisher 344 (F344) rats, weighing 180-210 g, and C57BL/6 mice, weighing 18-22 g, were obtained from Clea Japan (Tsukuba, Japan). Congenital AT-deficient mice (AT+/-) were kindly provided by Dr. Kojima, Nagoya University. Plasma levels of AT in AT+/- mice were about 50% of that in wild type[17]. Care and handling of the animals were in accordance with the National Institute of Health guidelines and the animals were fed standard animal chow and water. All the animal experiments were carried out in a humane manner after receiving permission from the Animal Experiment Committee of the university and in accordance with the Regulation of Animal Experiment and Japanese Governmental Law.
RCN-H4 cells, derived from hepatic metastasis of a F344 rat colon adenocarcinoma cell line, were provided by RIKEN Cell Bank (Tsukuba, Japan)[18,19]. RCN-H4 cells were maintained in RPMI 1640 supplemented with 100 mL/L heat inactivated FBS, 100 kU/L penicillin G and 100 mg/L streptomycin in a humidified 950mL/L air-50 mL/L CO2 at 37 °C. B16-F10 melanoma cells, which are syngeneic to C57BL/6 mice, were gifted from Cell Resource Center of Tohoku University (Sendai, Japan). B16-F10 cells were maintained in the same medium that was used for RCN-H4 cell culture.
Under anesthesia with diethyl ether, F344 rats underwent laparotomy. Warm ischemia of the median and left hepatic lobes was induced by clamping the left branches of the portal vein and the hepatic artery. The right lobe was perfused to prevent intestinal congestion. During the period of hepatic ischemia, the rat’s abdomen was covered with a plastic wrap to prevent dehydration. AT (500 U/kg) and OP-2507 (3 µg/kg) were dissolved in double distilled water, and administered intravenously 20 min after ischemia. Argatroban (1 mg/kg) and IM (5 mg/kg) were administered subcutaneously 30 min prior to ischemia. After 30 min of ischemia, the clamp was removed and the right lobe and caudate lobe were resected to prevent shunting to them after reperfusion with 4-0 silk. After resection of the right and caudate lobes, RCN-H4 cells (2×106 cells/rat) were intrasplenically administrated using a 30-gauge needle and the wound was closed with 3-0 silk. Sham-operated animals underwent the same operation but without clamping. During the surgical operation, the rat’s body temperature was kept between 35.5 °C and 36.5 °C by using a heating pad. The rates of breathing and heart beating were stable during the operation. None of the animals died until the 7th day after the operation.
The same surgical procedure as for F344 rats was performed for every mouse except for the anesthesia method (ketamine, 75 mg/kg body weight plus xylazine, 25 mg/kg body weight) and ischemia time (60 min). After resection of the right and the caudate lobes, B16-F10 cells (2×105 cells/mouse) were intrasplenically administrated. AT (500 U/kg) was administered 10 min before reperfusion. None of the animals died until the 7th day after the operation.
Animals were killed on the 7th day after the operation. The liver was removed, fixed with 10% buffered formalin for a few days, and cut into slices, each about 4 mm thick serially. These slices were stained with hematoxylin-eosin. The number of metastatic nodules was counted under a dissecting microscope (Olympus, Tokyo, Japan) at a magnification of ×100. The liver slice area was calculated with IP Lab software (Solution Systems Co., Ltd.) on a Macintosh computer. The number of metastatic nodules was normalized by the slice area (nodule number/cm2 ).
Ninety minutes after reperfusion, blood samples were collected in tubes containing 0.1 volume of 38g/L sodium citrate. Blood was centrifuged at 2 000 r/min for 10 min. Serum levels of fibrinogen degradation products (FDP (E)) were measured by a latex agglutination assay, as previously described[20].
Levels of 6-keto-PGF1α, a stable metabolite of PGI2, in liver tissue were measured as described previously[15,21]. In brief, tissue samples were weighed and homogenized in 0.1 mol/L phosphate buffer (pH 7.4) containing 0.5 g/L sodium azide. The homogenate was centrifuged (2 000 r/min, at 4 °C for 10 min) and the supernatant was stored at -80 °C until measurement. The concentration of 6-keto-PGF1α was determined with a specific enzyme immunoassay kit (Amersham, Buckinghamshire, UK). The results were expressed as nanograms of 6-keto-PGF1α per gram tissue.
Total RNA was isolated after homogenization of the tissue samples in TRIzol reagent according to the manufacturer,s instructions. RNA was reverse transcribed into cDNA in a 20-µL reaction volume containing 2 µL RNA (1 µg) in RNase-free water, 2 µL of 10× RT buffer, 4.4 µL of 25 mmol/L MgCl2, 4 µL deoxy NTPs mixture, 1 µL random hexamers, 0.4 µL RNase inhibitor, and 0.5 µL multiscribe reverse transcriptase (50 MU/L). Reverse transcription was performed at 25 °C for 10 min, at 37 °C for 60 min, and at 95 °C for 5 min, followed by quick chilling on ice and storage at 4 °C until subsequent amplification.
Real-time quantitative RT-PCR analysis was performed in triplicate using the ABI PRISM 7700 Sequence Detection System instrument and software (Applied Biosystems, Foster City, CA, USA). The primers and probes for the TaqMan system were designed using Primer Express software (Applied Biosystems) and synthesized using PE ABI (Weiterstadt, Germany). The 5,-end nucleotide of the probe was labeled with a reporter dye (FAM). The sequences of the PCR primer sets and probes used for each gene were purchased from TaqMan Gene Expression Assays (Applied Biosystems). The reaction conditions and PCR cycles were set according to the manufacturer’s directions.
All data were expressed as mean±SD. The results were compared using an analysis of variance (ANOVA) followed by Scheffe’s test. An associated probability of P<0.05 was considered to be statistically significant.
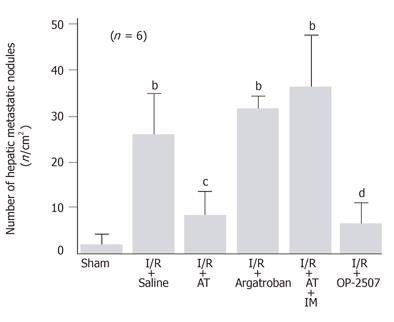
When intrasplenically injected, colon cancer cells formed a significant number of metastatic nodules in the liver of rats subjected to hepatic I/R on d 7 after the injection of cancer cells. The number of metastatic nodules in animals subjected to hepatic I/R was 13.6-fold than that observed in sham-operated animals (Figure 1). AT significantly inhibited the increase in the number of metastatic nodules in animals subjected to hepatic I/R (Figure 1). In contrast, argatroban, a synthetic selective inhibitor for thrombin, had no effect on this increase (Figure 1).
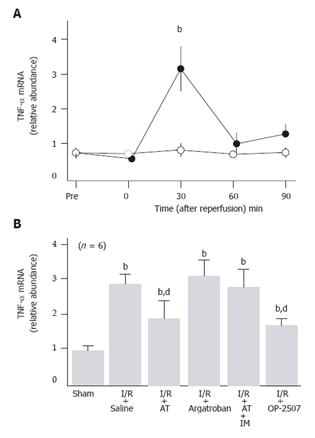
Hepatic tissue levels of TNF-α mRNA did not significantly increase in sham-operated animals, while these significantly increased 30 min after hepatic I/R and decreased thereafter to pre-ischemia levels (Figure 2A). AT significantly inhibited the I/R-induced increases in the hepatic tissue TNF-α mRNA levels, whereas argatroban did not inhibit these increases (Figure 2B).
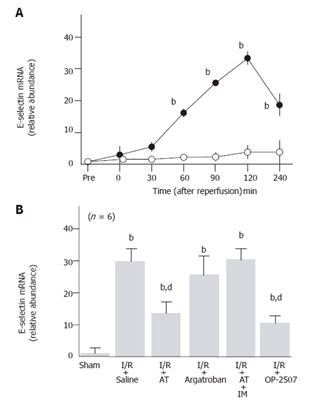
Although hepatic tissue levels of E-selectin mRNA did not increase in sham-operated animals, these levels began to increase 60 min after reperfusion, peaking at 120 min after reperfusion in animals subjected to hepatic I/R (Figure 3A). AT significantly inhibited the I/R-induced increases of E-selectin mRNA levels observed in hepatic tissue 120 min after reperfusion, whereas argatroban did not inhibit these increases (Figure 3B).
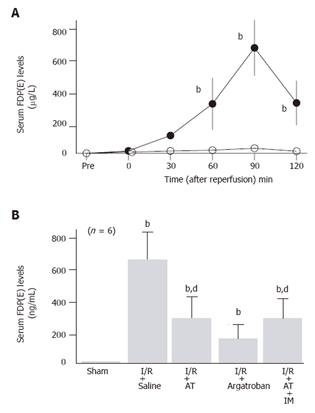
Serum levels of FDP(E) significantly increased after reperfusion, peaking 90 min after reperfusion in animals subjected to hepatic I/R, while these levels did not increase in sham-operated animals (Figure 4A). Both AT and argatroban inhibited the hepatic I/R-induced increases of serum FDP(E) observed 90 min after reperfusion (Figure 4B).
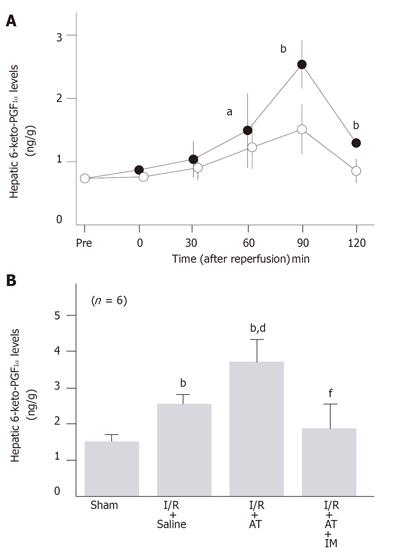
To examine whether AT reduced the hepatic I/R-induced increase in the number of metastatic nodules by promoting the endothelial release of PGI2, we have analyzed the effect of AT on the hepatic tissue levels of 6-keto-PGF1α after reperfusion. Hepatic tissue levels of 6-keto-PGF1α increased after reperfusion, peaking 90 min after reperfusion (Figure 5A). These levels were significantly higher in the I/R group than in sham-operated animals (Figure 5B). Administration of AT significantly enhanced the I/R-induced increases in hepatic levels of 6-keto-PGF1α 90 min after reperfusion, whereas argatroban did not (Figure 5B).
The anti-metastatic effect of AT was not observed in animals pretreated with IM which is known to inhibit cyclooxygenases (Figure 1). AT did not inhibit hepatic I/R-induced increases of TNF-α and E-selectin mRNA in hepatic tissue from animals pretreated with IM (Figures 2B and 3B). Hepatic I/R-induced increases in serum levels of FDP(E) were inhibited by AT in animals pretreated with IM (Figure 4B). Pretreatment of animals with IM significantly inhibited increases in hepatic tissue levels of 6-keto-PGF1α in animals given AT 90 min after reperfusion (Figure 5B).
To determine whether PGI2 inhibited I/R-induced increase in the number of hepatic metastases, we have examined the effect of OP-2507 on the I/R-induced increase in the number of hepatic metastatic nodules. As shown in Figure 1, the increase in the number of metastatic nodules in animals subjected to hepatic I/R was significantly inhibited by OP-2507. OP-2507 inhibited the I/R-induced increases in hepatic tissue levels of TNF-α and E-selectin mRNA observed 30 and 120 min after reperfusion, respectively (Figure 2B and 3B).
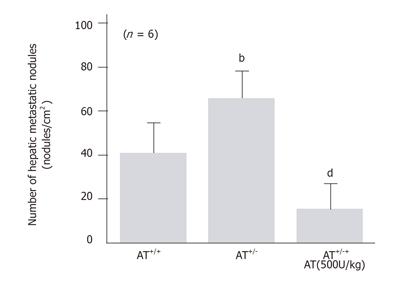
To know whether endogenous AT also inhibited hepatic metastasis of B16-F10 cells injected intrasplenically, we have compared the number of hepatic metastatic nodules observed after I/R in congenital AT-deficient mice (AT+/-) with that observed in wild-type C57BL/6J mice. The hepatic I/R-induced increase in the number of hepatic metastatic nodules observed 7 d after reperfusion was significantly higher in congenital AT+/- mice than in wild type mice (Figure 6). AT decreased the number of hepatic metastatic nodules in AT+/ mice subjected to hepatic I/R (Figure 6).
In the present study, AT reduced the hepatic I/R-induced increase of hepatic metastasis in rats to which colon cancer cells were intrasplenically injected. TNF-α is a pro-inflammatory cytokine that increases the expression of leukocyte adhesion molecules such as E-selectin, thereby contributing to the lodgment of cancer cells on the sinusoidal surface, leading to metastatic nodule formation[22]. AT inhibited hepatic I/R-induced increases in hepatic tissue levels of TNF-α and E-selectin mRNA, suggesting that AT might inhibit the hepatic I/R-induced increase of colon cancer cells metastases in our rat model by inhibiting TNF-α production. TNF-α plays a critical role in microthrombus formation in hepatic I/R[23]. Since thrombin increases the expression of E-selectin by activating protease-activated receptor-1 on endothelial cells[24], AT might inhibit the hepatic metastasis of colon cancer cells by inhibiting thrombin activity. However, this possibility seemed less likely, since argatroban, a selective inhibitor of thrombin that inhibited hepatic I/R-induced increases of serum FDP (E) to the same extent as AT, did not inhibit the development of hepatic metastases in the present study. We have previously reported that AT inhibits hepatic I/R-induced increases in hepatic tissue levels of TNF-α by promoting the I/R-induced increase in endothelial production of PGI2[16]. Consistent with this report is the present observation demonstrating that AT enhanced the hepatic I/R-induced increases in hepatic tissue levels of 6-keto-PGF1α. Pretreatment with IM reversed the effects of AT, including the inhibition of metastasis and increases in hepatic tissue levels of both TNF-α and E-selectin mRNA, suggesting that AT might inhibit hepatic I/R-induced increase in the metastasis of colon cancer cells by increasing endothelial production of PGI2. Furthermore, OP-2507, a stable derivative of PGI2, showed effects similar to those of AT, supporting the hypothesis described above.
Hepatic metastasis of intrasplenically injected cancer cells was significantly increased in heterozygous congenital AT-deficient mice whose plasma AT level is about half that of wild type mice[17] and replacement of AT in congenital AT-deficient mice reversed this increase in hepatic metastasis in the present study. These observations suggest that endogenous AT as well as AT given intravenously might be critically involved in the inhibition of hepatic I/R-induced increase of hepatic metastases in our experimental model.
Although the findings of the present study strongly suggest that AT might inhibit the metastasis of colon cancer cells via the promotion of endothelial production of PGI2, it is possible that a direct inhibition of tumor cell growth by AT also contribute to the inhibition of hepatic I/R-induced increase of colon cancer cell metastases. Our preliminary experiments using the monotetrazolium assay demonstrated that AT (5 kU/L) significantly inhibits the growth of RCN-H4 cells after incubation for 48 h[25]. Therefore, although IM pretreatment abrogated the anti-metastatic effect of AT in the present study, a direct effect of AT on cancer cell growth might contribute, at least in part, to the anti-metastatic effect of AT. This possibility should be further examined in future experiments.
AT concentrates are currently available for the treatment of disseminated intravascular coagulation and thrombosis in the clinical setting[26-28]. The findings of the present study raise the possibility that AT concentrates can effectively prevent hepatic metastasis of cancer cells during hepatectomy. These possibilities should be further examined in the clinical setting in the near future.
Prof T Kojima, Department of Medical Technology, Nagoya University School of Health Sciences for providing AT-deficient mice.
S- Editor Wang XL L- Editor Elsevier HK E- Editor Li HY
| 1. | Arnoletti JP, Brodsky J. Reduction of transfusion requirements during major hepatic resection for metastatic disease. Surgery. 1999;125:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Capussotti L, Massucco P, Ribero D, Viganò L, Muratore A, Calgaro M. Extended lymphadenectomy and vein resection for pancreatic head cancer: outcomes and implications for therapy. Arch Surg. 2003;138:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Ku Y, Kusunoki N, Shiotani M, Maeda I, Iwasaki T, Tominaga M, Kitagawa T, Fukumoto T, Suzuki Y, Kuroda Y. Stimulation of haematogenous liver metastases by ischaemia-reperfusion in rats. Eur J Surg. 1999;165:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Alexiou D, Karayiannakis AJ, Syrigos KN, Zbar A, Kremmyda A, Bramis I, Tsigris C. Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. Eur J Cancer. 2001;37:2392-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Ye C, Kiriyama K, Mistuoka C, Kannagi R, Ito K, Watanabe T, Kondo K, Akiyama S, Takagi H. Expression of E-selectin on endothelial cells of small veins in human colorectal cancer. Int J Cancer. 1995;61:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Uotani H, Yamashita I, Nagata T, Kishimoto H, Kashii Y, Tsukada K. Induction of E-selectin after partial hepatectomy promotes metastases to liver in mice. J Surg Res. 2001;96:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Wittig BM, Kaulen H, Thees R, Schmitt C, Knolle P, Stock J, Meyer zum Büschenfelde KH, Dippold W. Elevated serum E-selectin in patients with liver metastases of colorectal cancer. Eur J Cancer. 1996;32A:1215-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Kitagawa T, Matsumoto K, Iriyama K. Serum cell adhesion molecules in patients with colorectal cancer. Surg Today. 1998;28:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Burke J, Zibari GB, Brown MF, Granger N, Kelly R, Singh I, McDonald JC. Hepatic ischemia-reperfusion injury causes E-selectin upregulation. Transplant Proc. 1998;30:2321-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Rosenberg RD. Biochemistry of heparin antithrombin interactions, and the physiologic role of this natural anticoagulant mechanism. Am J Med. 1989;87:2S-9S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Uchiba M, Okajima K, Murakami K, Okabe H, Takatsuki K. Attenuation of endotoxin-induced pulmonary vascular injury by antithrombin III. Am J Physiol. 1996;270:L921-L930. [PubMed] |
| 13. | Oh-ishi S, Utsunomiya I, Yamamoto T, Komuro Y, Hara Y. Effects of prostaglandins and cyclic AMP on cytokine production in rat leukocytes. Eur J Pharmacol. 1996;300:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271:20828-20835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 289] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Harada N, Okajima K, Kushimoto S, Isobe H, Tanaka K. Antithrombin reduces ischemia/reperfusion injury of rat liver by increasing the hepatic level of prostacyclin. Blood. 1999;93:157-164. [PubMed] |
| 16. | Harada N, Okajima K, Uchiba M, Kushimoto S, Isobe H. Antithrombin reduces ischemia/reperfusion-induced liver injury in rats by activation of cyclooxygenase-1. Thromb Haemost. 2004;92:550-558. [PubMed] |
| 17. | Ishiguro K, Kojima T, Kadomatsu K, Nakayama Y, Takagi A, Suzuki M, Takeda N, Ito M, Yamamoto K, Matsushita T. Complete antithrombin deficiency in mice results in embryonic lethality. J Clin Invest. 2000;106:873-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Inoue Y, Kashima Y, Aizawa K, Hatakeyama K. A new rat colon cancer cell line metastasizes spontaneously: biologic characteristics and chemotherapeutic response. Jpn J Cancer Res. 1991;82:90-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Okuno K, Hirai N, Lee YS, Kawai I, Shigeoka H, Yasutomi M. Involvement of liver-associated immunity in hepatic metastasis formation. J Surg Res. 1998;75:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | CLAUSS A. [Rapid physiological coagulation method in determination of fibrinogen]. Acta Haematol. 1957;17:237-246. [PubMed] |
| 21. | Ramos E, Closa D, Hotter G, Roselló-Catafau J, Gelpi E, Fernandez-Cruz L. The impact of arterialization on prostanoid generation after liver transplantation in the rat. Transplantation. 1994;58:140-144. [PubMed] |
| 22. | Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71:612-619. [PubMed] |
| 23. | Okajima K, Harada N, Kushimoto S, Uchiba M. Role of microthrombus formation in the development of ischemia/reperfusion-induced liver injury in rats. Thromb Haemost. 2002;88:473-480. [PubMed] |
| 24. | Shankar R, de la Motte CA, Poptic EJ, DiCorleto PE. Thrombin receptor-activating peptides differentially stimulate platelet-derived growth factor production, monocytic cell adhesion, and E-selectin expression in human umbilical vein endothelial cells. J Biol Chem. 1994;269:13936-13941. [PubMed] |
| 25. | Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38285] [Cited by in RCA: 39418] [Article Influence: 938.5] [Reference Citation Analysis (0)] |
| 26. | Fourrier F, Chopin C, Huart JJ, Runge I, Caron C, Goudemand J. Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest. 1993;104:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 219] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Inthorn D, Hoffmann JN, Hartl WH, Mühlbayer D, Jochum M. Antithrombin III supplementation in severe sepsis: beneficial effects on organ dysfunction. Shock. 1997;8:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Bucur SZ, Levy JH, Despotis GJ, Spiess BD, Hillyer CD. Uses of antithrombin III concentrate in congenital and acquired deficiency states. Transfusion. 1998;38:481-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |