Published online Jan 7, 2006. doi: 10.3748/wjg.v12.i1.29
Revised: May 28, 2005
Accepted: June 11, 2005
Published online: January 7, 2006
AIM: Polo-like kinase 1 (PLK1) serine/threonine kinase plays a vital role in multiple phases of mitosis in gastric cancer cells. To investigate the effect of PLK1 depletion on mitosis and apoptosis of gastric cancer cells.
METHODS: PLK1 expression was blocked by small RNA interference(siRNA). The expression levels of PLK1, cdc2, cyclin B and caspase 3 were detected by Western blotting. Then, PLK1 depletion, cdc2 activity, cell proliferation, cell cycle phase distribution, mitotic spindle structure, and the rate of apoptosis of the PLK1 knockdown cells were observed.
RESULTS: PLK1 gene knockdown was associated with increased cyclin B expression, increased cdc2 activity (but not with the expression levels), accumulation of gastric cancer cells at G2/M, improper mitotic spindle formation, delayed chromosome separation and delayed or arrested cytokinesis. Moreover, PLK1 depletion in gastric cancer cells was associated with decreased proliferation, attenuated pro-caspase 3 levels and increased apoptosis.
CONCLUSION: Blockage of PLK1 expression may lead to decreased mitosis or even apoptosis in gastric cancer cells, indicating that PLK1 may be a valuable therapeutic target for gastric cancer.
- Citation: Chen XH, Lan B, Qu Y, Zhang XQ, Cai Q, Liu BY, Zhu ZG. Inhibitory effect of Polo-like kinase 1 depletion on mitosis and apoptosis of gastric cancer cells. World J Gastroenterol 2006; 12(1): 29-35
- URL: https://www.wjgnet.com/1007-9327/full/v12/i1/29.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i1.29
The separation of chromosomes and the division of one cell into two daughter cells are the most dramatic events during the cell cycle. This process, known as mitosis, involves a series of structural changes in cells, including centrosome duplication, spindle formation and cytokinesis. The cdc2 - cyclinB protein complex, a mitosis promoting factor , is known to trigger and promote the completion of this complex process[1], but the mechanism responsible for activating the cdc2 - cyclinB complex remained unknown until the conserved Polo-like kinase 1 (PLK1) was cloned by Golsteyn et al.[2]. PLK1 accumulates markedly during the G2/M phase in cells, where it phosphorylates and activates the cdc2 - cyclinB complex and other mitosis-involved substrates[3,4]. PLK1 is indispensable in some types of cell division including tumor cells[5]. Besides, PLK1 expression increases in multiple types of solid tumors such as non-small-cell lung cancer, head and neck cancer, esophageal cancer, colorectal cancer-associated glioma[6-10]. PLK1 is now considered as an oncogene, a potential target for cancer therapy. But to our knowledge, no previous work has examined the effect of PLK1 knockdown in gastric cancer cells. Here, we have used RNA interference technology to block the expression of PLK1 in gastric cancer cell line MKN45 and observed the changes in cell division phenotypes and cell viability.
The gastric cancer cell line MKN45 was maintained in RPMI1640 medium containing 2 mmol/L glutamine and 100ml/L fetal bovine serum (Invitrogen, CA, USA) and incubated at 37 °C in an atmosphere containing 50 mL/L of CO2.
The Ambion software was used to design RNAi sequences targeting human PLK1 (accession no. NM 005030) and the siRNA sequence with the highest putative efficacy (5’CAACCAAAGTCGAATATGA 3’) was synthesized by Shanghai GeneChem Co., Ltd. (PLK– group). Small RNA interference with randomized sequence (5’TTCTCCGAACGTGTCACGT3’) against no gene (scrambled siRNA group) and only liposome (liposome group) were transfected as internal control.
For the experiments, cells transfected with the DOTAP liposomal transfection reagent (Roche, Germany) were seeded at 2 × 105 cells/well in six-well plates. After 24 h culture when cells were in the phase of log growth, 250 μL Opti-MEM I was mixed with 7.5 μL of 20 µmol/L siRNA duplex, while another 250 μL Opti-MEM I was separately incubated with 11.88 μL of DOTAP liposome. The two mixtures were gently mixed and incubated for about 30 min at room temperature. For transfection, the entire mixture was added to each well in 1.5 mL of fresh medium containing 100 ml/L FBS without antibiotics. The final transfected concentration of siRNA was 75 nmol/L. Cells were collected for further assay at 24, 48, and 72 h after transfection.
Cells were lysed in AM1 lysis buffer (Active Motif, USA) and protein concentrations were measured with the BCA protein assay kit (Pierce, USA). Total protein (50 μg) was resolved by 125 g/L SDS-PAGE and transferred onto PVDF membranes. After being blocked in TBST (20 mmol/L Tris, 137 mmol/L NaCl, 1 g/L Tween 20, pH 7.6) with 50 ml/L skim milk for 2 h at room temperature, membranes were incubated with PLK1, cyclinB, cdc2, pro-caspase 3, and β-actin primary antibodies (diluted 1:200; Santa Cruz Biotechnology, USA) for 2 h. Membranes were then washed thrice with TBST solution, followed by incubation for 1 h with HRP-linked secondary antibodies (1:1 000; Santa Cruz Biotechnology) at room temperature. Finally, membranes were visualized using the DAB reagent (Dako Corporation, Denmark).
Cdc2 kinase activity was measured using the cdc2-cyclinB kinase assay kit (MBLTM International, Japan) according to the manufacturer’s instructions.
Cells were harvested by trypsin, washed with cold PBS and resuspended in 750 ml/L ethanol at 4 °C for at least 8 h. The fixed cells were collected by brief centrifugation and resuspended in PBS containing 200 µg/mL RNase and 15 mg/L propidium iodide. After incubation at room temperature for 30 min, samples were subjected to flow cytometry (FCM) for Fluorescence-activated cell sorting assay and cell cycle phase analysis.
Cells were grown on coverslips, fixed with 40g/L paraformaldehyde for 10 min and permeabilized with methanol for 2 min. After being washed thrice with 1 Triton X-100 in PBS, the coverslips were blocked with 20 ml/L FBS for 30 min, stained with 10 mg/L anti-α-tubulin primary antibody for 2 h at room temperature and incubated with FITC-conjugated secondary antibody (Santa Cruz Biotechnology) for 30 min. Finally, the DNA was stained with TO - PRO - 3 iodide (Molecular Probes, USA) and confocal microscopy was performed to obtain detailed images of the subcellular structures.
The MTT method was used to measure cell proliferation. Briefly, 4 × 103 cells/well were cultured in 96 - well plates. After 24 h, siRNA and liposome were added to transfect cells, and then at the designated time points (24, 48, 72, and 96 h after transfection), MTT (5 g/L) was added. Cells were incubated for 4 h and then the medium was removed and 100 μL DMSO solution was added for 5 min. The absorbance of the reaction solution was measured at 570 nm and the data from the various time points were used to generate cell proliferation curves.
Apoptosis was assessed with an Annexin V kit (BD Corporation, USA). In brief, cells were harvested, washed in PBS and labeled with Annexin V antibody and PI according to the manufacturer’s protocol. Labeled cells were then subjected to FCM for the determination of apoptosis.
All experiments were repeated thrice. Data were analyzed with SPSS software version 11.0. Student’s t-test was used to evaluate the difference in cell phase distribution between PLK– and scrambled siRNA groups. One-way analysis of variance (ANOVA) was used to test the effects of the three treatments on cdc2 activity and apoptosis rate. Two-way analysis of variance was performed to detect the random effects of the three treatments on cell proliferation (MTT assay), and Student-Newman-Keuls test was used to detect the difference between any two groups. The χ2 test was employed to examine the difference in cell mitosis phenotypes under confocal microscope. Cell morphology was observed under inverted microscope between experimental and control groups. P < 0.05 was considered statistically significant.
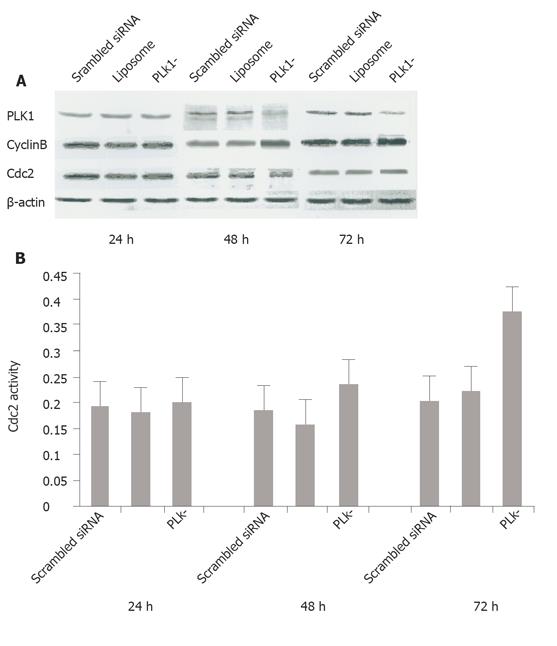
To determine the effect of siRNA on PLK1 depletion, we collected cell samples at 24, 48, and 72 h after transfection, extracted total cellular proteins and performed standard Western blotting of experimental cultures and scrambled siRNA-treated and liposome-only controls. PLK1 protein levels were reduced by 38.4% and 60.7% compared with the scrambled siRNA-treated control groups at 48 and 72 h, respectively, indicating that PLK1 gene expression was obviously blocked by the siRNAs (Figure 1A). We found that the cyclinB protein levels were 82.4% and 32.9% higher at 48 and 72 h, respectively in PLK1-depleted cells than in scrambled siRNA-treated cells (Figure 1A), while the mean activity of cdc2 kinase was increased in comparison to the controls (85.71% and 68% higher than scrambled siRNA-treated cells at 48 and 72 h, respectively, P < 0.05), but there was no apparent difference at the protein level (Figures 1A and 1B).
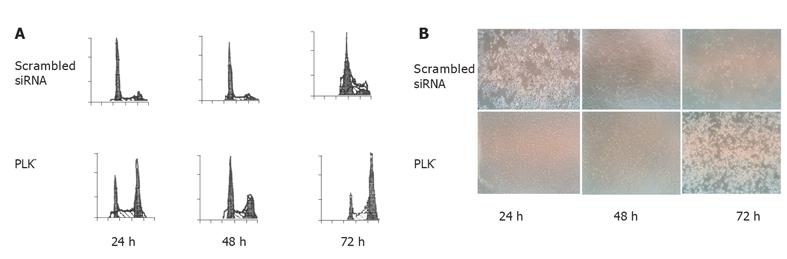
Cell cycle phase distribution was measured by FCM at 24, 48, and 72 h after siRNA transfection. The mean percentage of cells with G2 DNA content in the PLK– group was 40.15%, 36.58%, and 59.88%, while that in the scrambled siRNA control group was 14.59%, 10.11%, and 17.69% at 24, 48, and 72 h, respectively (P < 0.05, Figure 2A). Inverted microscopy was used to examine the morphological changes of tumor cells under five randomly chosen inverted microscopic fields. The percentage of rounded cells was higher in the experimental group than in the scrambled siRNA control group (41% vs 13%, 38.55% vs 13.21%, and 44.55% vs 18.2% at 24, 48, and 72 h, respectively) (P < 0.05, Figure 2B).
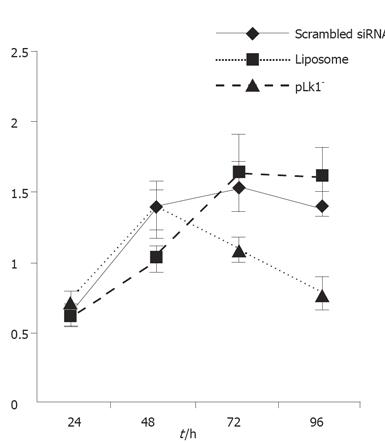
MTT assays were used to determine whether PLK1 gene depletion affected tumor cell proliferation. The resultant proliferation curves indicated that the PLK1-depleted cells divided more slowly than cells in the two control groups (P < 0.05, Figure 3).
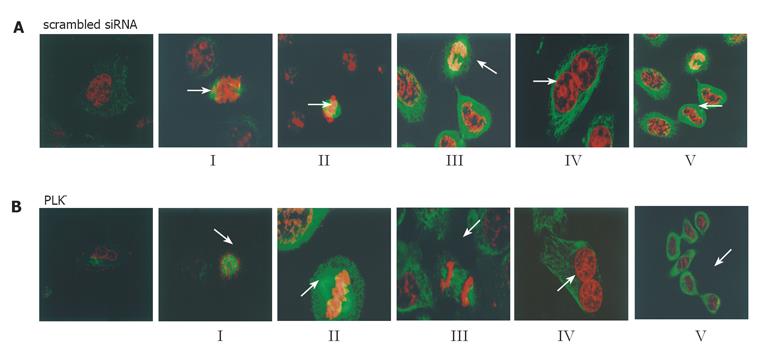
Changes in the mitotic phenotypes of PLK1 knockdown and control cells were observed by immunofluorescence staining and confocal microscopy. The spindle structure lost its cohesiveness 48 h after siRNA transfection (Figure 4A). Images of the five different substages of mitosis could be acquired (Figure 4B). There were substantial differences in the amounts and percentages of cells between the experimental group and scrambled siRNA control group after 48 h (P < 0.05, Table 1) at the onset of mitosis. More PLK– cells (46% vs 20%) were at substage I (nuclear membrane breakdown and even chromosomal distribution in the cytoplasm) and fewer (1% vs 41%) were at substage II (chromosomal array along the equator plate) and III (2% vs 8%) (chromosomal segregation). Meanwhile, higher percentages of cells with dumbbell-shaped nuclei (33% vs 15%) and cytoplasmic bridges connecting two incompletely separated cells (8% vs 1%) were also shown in PLK– cells. These results collectively indicated that spindle formation, chromosome separation and cytokinesis were delayed during mitosis in MKN45 cells transfected with PLK1-specific siRNA duplexes.
| I | II | III | IV | V | Other types | Total | |
| Scrambled siRNA | 20 | 41 | 8 | 15 | 1 | 15 | 100 |
| PLK– | 46 | 1 | 2 | 33 | 8 | 10 | 100 |
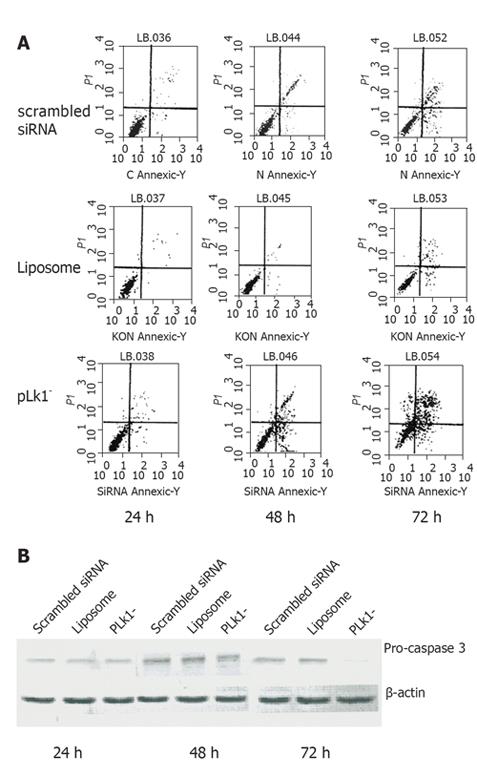
To evaluate the role of PLK1 in tumor cell fate, PLK1-depleted cells were labeled with Annexin V antibody and subjected to FCM. The results showed that the mean apoptosis rate (including early and late apoptosis) at 48 and 72 h was higher in PLK1-depleted cells than in scrambled siRNA cells (42.4% vs 21.4%, 53.8% vs 32.9%, P < 0.05, Figure 5A). Furthermore, Western blotting revealed that caspase 3 level was lower in PLK1-depleted cells (Figure 5B).
Gastric cancer is one of the most common malignancies in the world. Uncontrolled cellular proliferation is commonly associated with the poor prognosis of this disease. Human PLK1 plays a key role in some mitotic events[11,12]. Its expression is associated with tumor proliferation degree[13]. PLK1 depletion is associated with HeLa cell mitosis[5]. However, no previous work has examined the possible role of PLK1 in gastric cancer cell mitosis and cell fate. In this experiment, we have used siRNA technology to knockdown PLK1 gene expression and observed the changes in mitotic phenotypes of the gastric cancer cell line, MKN45.
The protein level of PLK1 was decreased by 38.4% and 60.7%, respectively at 48 and 72 h after the addition of the PLK1-specific siRNA, indicating that the interference is effective and this system is appropriate for the study of mitotic events under PLK1 knockdown conditions. A previous study has demonstrated that the direct motivation of driving cells from G2 to M phase is caused by the cyclinB-cdc2 complex (also known as the mitosis promoting factor or MPF). PLK1 is responsible for phosphorylating and activating MPF at the G2/M checkpoint[3] and mediates the degradation of cyclinB by activating the anaphase-promoting complex at the end of mitosis[14,15], indicating that PLK1 can initiate and maintain mitosis. In our PLK1 knockdown cells, the cyclinB level increased probably due to the failure of depleted PLK1 to activate APC. In addition, the activity (but not the quantity) of cdc2 (a component of MPF) was increased by 85.7% and 68.0% at 48 and 72 h, respectively in PLK1 knockdown cells compared to scrambled siRNA controls. Previous reports indicate that PLK1 and its analogs seem to phosphorylate and activate cdc25[3,16], which then dephosphorylates and activates the cdc2/cyclinB complex in different organisms[17,18], suggesting that cdc2 may lose its activity under PLK1 knockdownconditions. Our observation of cdc2 activation seems to indicate that cdc2 is activated in a cdc25-independent manner.
Immunofluorescence and confocal microscopy revealed that the microtubule morphology was affected in PLK1-depleted cells, becoming broken and unclear. We were able to identify cells in both PLK1-depleted and control cultures corresponding to the five substages of mitosis: (I) nuclear membrane breakdown and even chromosomal distribution in cytoplasm; (II) chromosomal array along the equator plate; (III) chromosomal segregation; (IV) mitotic exit and nuclear membrane formation; (V) cytokinesis. These substages were observed at varying frequencies between the control and siRNA-treated cells. At the onset of mitosis, more siRNA-treated cells (46% vs 20%) were at substage I and fewer were at substage II (1% vs 41%) or III (2% vs 8%). Recent studies showed that PLK1 affects chromosomal separation by controlling the formation of mitotic spindle and phosphorylating cohesion to decrease the cohesion of sister chromosomes[19-21]. Our results revealed that in PLK1-depleted tumor cells, the mitotic spindle was disrupted (Figure 4A), which may be the reason why many PLK1-depleted cells are stalled in substage I and unable to progress to the subsequent stages requiring intact mitotic spindles.
In addition to its effect on chromosomal segregation, PLK1 is also closely associated with the exit from mitosis and subsequent cytokinesis. In the former, PLK1 appears to play a role in the activation of APC by destroying the APC inhibitor, Emi1[15,22]. In cytokinesis, PLK1 is associated with the phosphorylation and activation of motor-like protein (MKlp) 2[23] and nuclear distribution gene C (NudC)[24,25]. In our study, 48 h after siRNA treatment, α-tubulin immunofluorescence and DNA staining revealed a higher percentage of dumbbell-shaped cell nucleoli (33% vs 15%) and increased the number of cytoplasmic bridges connecting two incompletely separated cells (8% vs 1%), indicating that PLK1-depleted cells cannot successfully exit mitosis and reform two new nuclear membranes at anaphase but arrest mitosis during cytokinesis. In our study, immunohistochemistry and FCM experiments showed that the expression of PLK1-specific RNAi affected mitotic processes, causing tumor cells to accumulate at the mitotic phase and blocking them from completing cell division. In addition, our MTT assays indicated that PLK1-depleted cells decreased their proliferation.
We examined whether PLK1 depletion affected the fate of MKN45 cells. We used Annexin V staining to identify early and late apoptosis. Our results revealed that apoptosis increased significantly 48 and 72 h after the addition of the PLK1-specific siRNA. There was no obvious change at 24 h, indicating a delay before the apoptotic mechanism is triggered. Further examination revealed a sharp decrease in pro-caspase 3 levels. PLK1 can bind to and suppress the pro-apoptotic molecule p53 in HeLa cells[26], while p53 is accumulated during PLK1-depletion-induced apoptosis[27]. Based on this, it may be interesting to clarify whether any other pathway is involved in PLK1 depletion-induced apoptosis.
In conclusion, PLK1 is vital to gastric cancer cell division. Gene or drug therapy aimed at depleting PLK1 level may have a potential value as a novel treatment for gastric cancer.
Bian Wei from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, for technical assistance with the confocal microscopy.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Wu M
| 1. | Ohi R, Gould KL. Regulating the onset of mitosis. Curr Opin Cell Biol. 1999;11:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Golsteyn RM, Schultz SJ, Bartek J, Ziemiecki A, Ried T, Nigg EA. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994;107:1509-1517. [PubMed] |
| 3. | Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 248] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Lee M, Daniels MJ, Venkitaraman AR. Phosphorylation of BRCA2 by the Polo-like kinase Plk1 is regulated by DNA damage and mitotic progression. Oncogene. 2004;23:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Liu X, Erikson RL. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc Natl Acad Sci U S A. 2002;99:8672-8676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, Altmannsberger HM, Rübsamen-Waigmann H, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 278] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 7. | Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794-2797. [PubMed] |
| 8. | Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999;15:687-692. [PubMed] |
| 9. | Takahashi T, Sano B, Nagata T, Kato H, Sugiyama Y, Kunieda K, Kimura M, Okano Y, Saji S. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci. 2003;94:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Dietzmann K, Kirches E, von Bossanyi K, Mawrin C. Increased human polo-like kinase-1 expression in gliomas. J Neurooncol. 2001;53:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Golsteyn RM, Lane HA, Mundt KE, Arnaud L, Nigg EA. The family of polo-like kinases. Prog Cell Cycle Res. 1996;2:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Mundt KE, Golsteyn RM, Lane HA, Nigg EA. On the regulation and function of human polo-like kinase 1 (PLK1): effects of overexpression on cell cycle progression. Biochem Biophys Res Commun. 1997;239:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Ito Y, Yoshida H, Matsuzuka F, Matsuura N, Nakamura Y, Nakamine H, Kakudo K, Kuma K, Miyauchi A. Polo-like kinase 1 (PLK1) expression is associated with cell proliferative activity and cdc2 expression in malignant lymphoma of the thyroid. Anticancer Res. 2004;24:259-263. [PubMed] |
| 14. | Kotani S, Tugendreich S, Fujii M, Jorgensen PM, Watanabe N, Hoog C, Hieter P, Todokoro K. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol Cell. 1998;1:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 234] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Moshe Y, Boulaire J, Pagano M, Hershko A. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc Natl Acad Sci U S A. 2004;101:7937-7942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Karaïskou A, Jessus C, Brassac T, Ozon R. Phosphatase 2A and polo kinase, two antagonistic regulators of cdc25 activation and MPF auto-amplification. J Cell Sci. 1999;112:3747-3756. [PubMed] |
| 17. | Karaïskou A, Cayla X, Haccard O, Jessus C, Ozon R. MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp Cell Res. 1998;244:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Abrieu A, Brassac T, Galas S, Fisher D, Labbé JC, Dorée M. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci. 1998;111:1751-1757. [PubMed] |
| 19. | Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, Peters JM. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Peters JM. Subunits and substrates of the anaphase-promoting complex. Exp Cell Res. 1999;248:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Sumara I, Giménez-Abián JF, Gerlich D, Hirota T, Kraft C, de la Torre C, Ellenberg J, Peters JM. Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr Biol. 2004;14:1712-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 268] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 22. | Hansen DV, Loktev AV, Ban KH, Jackson PK. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFbetaTrCP-dependent destruction of the APC Inhibitor Emi1. Mol Biol Cell. 2004;15:5623-5634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Neef R, Preisinger C, Sutcliffe J, Kopajtich R, Nigg EA, Mayer TU, Barr FA. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J Cell Biol. 2003;162:863-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 24. | Zhou T, Aumais JP, Liu X, Yu-Lee LY, Erikson RL. A role for Plk1 phosphorylation of NudC in cytokinesis. Dev Cell. 2003;5:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Aumais JP, Williams SN, Luo W, Nishino M, Caldwell KA, Caldwell GA, Lin SH, Yu-Lee LY. Role for NudC, a dynein-associated nuclear movement protein, in mitosis and cytokinesis. J Cell Sci. 2003;116:1991-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M, Nakagawara A. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem. 2004;279:25549-25561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci U S A. 2003;100:5789-5794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |