Published online Jan 7, 2006. doi: 10.3748/wjg.v12.i1.137
Revised: June 2, 2005
Accepted: June 6, 2005
Published online: January 7, 2006
AIM: To investigate the therapeutic effects of resveratrol (RESV) as a free radical scavenger on experimental severe acute pancreatitis (SAP).
METHODS: Seventy-two male Sprague–Dawley rats were divided randomly into sham operation group, SAP group, and resveratrol-treated group. Pancreatitis was induced by intraductal administration of 0.1 mL/kg 4% sodium taurocholate. RESV was given intravenously at a dose of 20 mg/kg body weight. All animals were killed at 3, 6, 12 h after induction of the model. Serum amylase, pancreatic superoxide dismutase (SOD), malondialdehyde (MDA), and myeloperoxidase (MPO) were determined. Pathologic changes of the pancreas were observed under optical microscope.
RESULTS: The serum amylase, pancreatic MPO and the score of pathologic damage increased after the induction of pancreatitis, early (3, 6 h) SAP samples were characterized by decreased pancreatic SOD and increased pancreatic MDA. Resveratrol exhibited a protective effect against lipid peroxidation in cell membrane caused by oxygen free radicals in the early stage of SAP. This attenuation of the redox state impairment reduced cellular oxidative damage, as reflected by lower serum amylase, less severe pancreatic lesions, normal pancreatic MDA levels, as well as diminished neutrophil infiltration in pancreas.
CONCLUSION: RESV may exert its therapeutic effect on SAP by lowering pancreatic oxidative free radicals and reducing pancreatic tissue infiltration of neutrophils.
- Citation: Li ZD, Ma QY, Wang CA. Effect of resveratrol on pancreatic oxygen free radicals in rats with severe acute pancreatitis. World J Gastroenterol 2006; 12(1): 137-140
- URL: https://www.wjgnet.com/1007-9327/full/v12/i1/137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i1.137
Resveratrol (3,5,4’-trihydroxystilbene) is a naturally occurring phytoalexin present in grapes, fruits, and a variety of medicinal plants[1]. It is the major active component of Rhubarb and Giant Knotweed Rhizome, etc., in traditional Chinese medicine. In in vitro, ex vivo, and in vivo experiments, RESV displays diverse pharmacological effects including modulation of lipoprotein metabolism and cardiovascular protection[2], anti-inflammation[3], platelet antiaggregatory activity[2], antimicrobial activity[4], antiallergic activity[5], anticancer properties[6,7], and most notably, antioxidant properties[8]. In the present study, the sodium taurocholate-induced model of SAP was used to investigate the effects of RESV on SOD, MDA, MPO, serum amylase, and pancreatic pathological change to assess the role of oxidative stress in SAP and the therapeutic effects of RESV on SAP.
RESV was obtained from Huike Botanical Development Co, stocked solution of RESV was made in Tween-80 at the concentration of 10 mg/mL and kept frozen. Sodium taurocholate was purchased from Sigma Chemical Co. SOD, MDA, and MPO assay reagents were from Nanjing Jiangcheng Bioengineering Institute.
Male Sprague-Dawley rats weighing 250 - 300 g, purchased from Laboratory Animal Center attached to Medical College of Xi’an Jiaotong University, were used. All animals were housed in a macroion cage at 22 - 24 °C in a 12/12-h light/dark cycle. The animals were given a standard rat chow and fasted overnight with free access to water before the experiment. Care was provided in accordance with the “Guide for the care and use of laboratory animals” (NIH publication No. 85 - 23, revised in 1996). The study was approved by the Subcommittee on Experimental Animal Care of our institution.
The rats were anesthetized by an intraperitoneal injection of pentobarbital (30 mg/kg, Sigma). Through a midline incision, the duodenum and the pancreatic bile duct were identified, and the duodenal wall was punctured with a 24-gauge Teflon catheter. The catheter was advanced into the common pancreatic bile duct. A microvascular clamp was placed on the duct at the hilum of the liver, a microtube clamp was placed around the catheter and the wall of the duct close to the duodenum to prevent reflux. Four percent sodium taurocholate (1 mL/kg body weight, Sigma T - 0750) was injected into the pancreatic bile duct for 60 s. The clamp remained in place throughout the intraductal infusion to prevent misdirected flow into the biliary system.
Seventy-two rats were randomly divided into sham operation group: laparotomy followed by tipping of the pancreas without any infusion, SAP group: receiving infusion of 40 g/L sodium taurocholate into the pancreatic bile duct, RESV-treated group: perfused with RESV at a dose of 20 mg/kg body weight through vena dorsalis penis 10 min after the induction of SAP. After 3, 6, and 12 h, eight rats from each group were killed and blood was taken from the left ventricle of the heart. The samples were centrifuged (3 000 r/min, 10 min, 4 ºC) and serum was derivatized and immediately stored at -70 °C for amylase determination. The pancreas was removed; the head of pancreas was fixed in 40 g/L paraformaldehyde for histologic analysis. Caudal pancreatic tissue was powdered using a mortar and pestle on dry ice and immediately stored at -70 °C for determining pancreatic SOD, MDA, and MPO.
Tissue samples of the pancreas were fixed in 40 g/L paraformaldehyde and embedded with paraffin. Five-micrometer thick sections were stained with hematoxylin/eosin and examined and graded as previously described[9]. The total surface of the slides was scored by one blinded pathologist for four different variables (edema, acinar necrosis, hemorrhage and fat necrosis, inflammation and perivascular infiltrate) to determine the severity of pancreatic injury.
Amylase activity in serum was determined using an automatic biochemistry analyzer (Hitachi 7170). Pancreas was homogenized in physiological saline or 5 g/L HTAB using ultrasonication. The SOD content was measured using the xanthine oxidase technique based on the spectrophotometric monitoring of SOD-mediated reduction of DTNB at 550 nm. The concentration of MDA was quantified by thiobarbituric acid reaction and MPO contents were determined as described by Bhatia et al [10].
Results were expressed as mean ± SE. Statistical analysis was done using the SPSS10.0 software package. One-way analysis of variance was used to establish whether the difference among the three groups was statistically significant. P < 0.05 was considered statistically significant.
There was no or a small amount of clear ascitic fluid in sham operation group. More than 8 mL turbid hemorrhagic ascites could be seen in all rats of SAP group. No obvious change or slight edema could be seen in sham operation group. Pancreas in SAP group displayed disparate edema with punctiform or lamellar hemorrhage and necrosis. Saponified spots could be seen at pancreas, epiploon, mesentery, peritoneum, and perinephric fat. Pancreatic tissue was normal in sham operation group. In SAP group, pancreatic tissue displayed interstitial edema, widened lobula interspace, inflammatory cell infiltration, hemorrhage and necrosis. Microthromb could be found inside the small vessels around focal necrosis of the pancreas. In contrast, in RESV-treated group, the ascitic fluid diminished significantly and turbidity was lower than that in SAP group. Saponified spots, pancreatic edema, necrosis, inflammatory cell infiltration decreased significantly in RESV-treated group (Table 1).
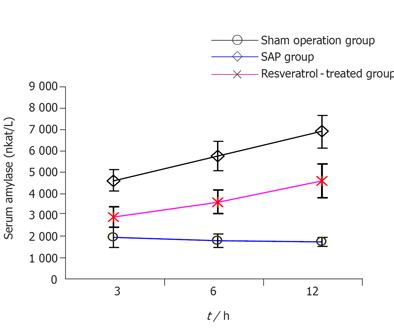
Compared to sham operation group, the serum amylase in SAP group increased at all time points (P < 0.01), decreased significantly in RESV-treated group when compared to SAP group at the corresponding time points (P < 0.01, Figure 1).
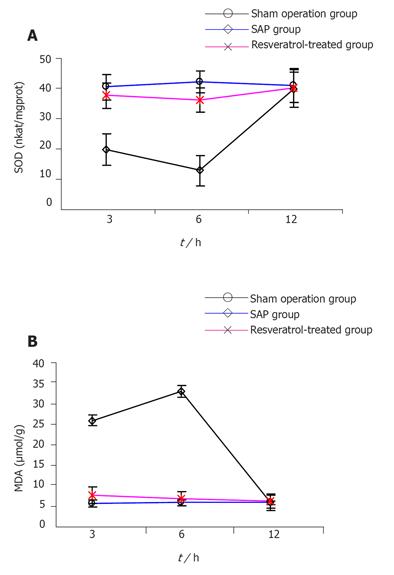
Compared to sham operation group, pancreatic SOD descended and MDA increased in SAP group at 3 and 6 h (P < 0.01), but there was no difference between the two groups at 12 h in SOD and MDA. In contrast, pancreatic SOD increased and MDA descended in RESV-treated group at 3 and 6 h when compared to SAP group (P < 0.01), but there was no difference in SOD and MDA at 12 h between two groups (Figures 2A and 2B).
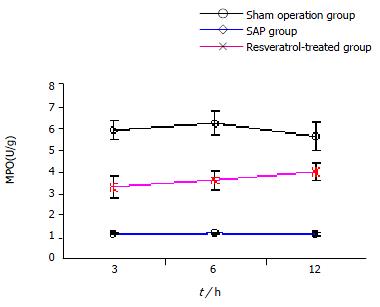
Compared to sham operation group, the pancreatic MPO in SAP group increased at all time points (P < 0.01), but decreased significantly in RESV-treated group when compared to SAP group at the corresponding time points (P < 0.01, Figure 3).
The pathogenesis and therapeutics of SAP are constantly emphasized in general surgery. Sanfey et al [11] have suggested a possible involvement of oxygen free radicals (OFRs) in acute pancreatitis. In 1995, Kishimoto et al.[12] detected pancreatic OFRs in acute pancreatitis using the technique of chemiluminescence probe and high sensitive photon counting and found that OFRs emerge 2 - 3 h after the induction of acute pancreatitis, demonstrating that there exists peroxidation in acute pancreatitis. OFRs can attack polyunsaturated fatty acid’s aldehyde group inside the biomembrane, initiating lipid peroxidation and accordingly forming lipid peroxidation products, as such MDA, which result in the loss of membrane stability and release of acinar cell enzyme precursors, and activate phospholipase A1 which can decompose lecithinum inside cellular membrane, further causing tissue damage. SOD is an internal antioxidase. OFRs in vivo are augmented when acute pancreatitis develops, which results in the consumption of antioxidant, SOD activity decrease. Therefore, it is difficult to prevent damage to the pancreas and other organs by lipid peroxidation. Detection of pancreatic SOD and MDA can reflect the peroxidation of pancreatic acinar cells and indirectly reflect the damage due to OFRs.
A number of antioxidant therapies can improve pancreatitis induced by the administration of cerulein[13] and infusion of taurocholate[14]. Lasztity et al.[15] found that when enteral formula enriched with n-3 polyunsaturated fatty acids is used in the treatment of acute pancreatitis, the erythrocyte SOD activity is elevated significantly. Leonard et al.[8] showed that RESV can scavenge OFRs as measured by spin trapping competitions using sodium formate as a second free radical scavenger, and is effective in inhibiting lipid peroxidation of cellular membranes. In the present study, when compared to sham operation group, 3 h after the induction of SAP, pancreatic SOD decreased significantly, reaching perigee at 6 h, and returned to the level of sham operation group at 12 h. In contrast, 3 h after the induction of SAP, pancreatic MDA increased significantly, reaching to apogee at 6 h, and returned to the level of sham operation group at 12 h. Simultaneously, the serum amylase and pancreatic histopatho-logic score increased gradually. The results indic-ate that overproduction of OFRs occurs in early SAP and is a significant factor for aggravating pathogenetic condition. This is coincident with the research by Reinheckel et al [16]. When compared to SAP group, pancreatic SOD in RESV-treated group increased significantly at 3 and 6 h (P < 0.01), whereas pancreatic MDA in RESV-treated group decreased significantly at 3 and 6 h (P < 0.01). On the other hand, compared to SAP group, both serum amylase and pancreatic histopathologic score in RESV-treated group decreased at all three time points (P < 0.01) indicating that RESV can depress earlier OFR production and lipid peroxidation of cellular membrane, diminish enzyme precursor release and necrosis of acinar cells, thus ameliorating pancreatic pathological lesions.
Neutrophils are the other major cellular source of OFRs during acute pancreatitis[17,18], and can directly release several inflammatory cytokines, evoking systemic inflammatory reactive syndrome (SIRS). since OFRs can exert a chemoattractant effect, thereby promoting accumulation of leukocytes in the inflamed gland[17]. Decreased acinar OFR production after RESV treatment may contribute to the reduced neutrophil infiltration, further ameliorating SIRS in SAP. In our study neutrophil sequestration within the pancreas was estimated by measuring tissue MPO activity. When compared to SAP group, the pancreatic MPO decreased at all the time points in RESV-treated group (P < 0.01). Moreover, studies showed that RESV can suppress the activation of NF-κB. Thus, RESV treatment might lead to the suppression of NF-κB activation and the subsequent prevention of several inflammatory mediator genes from being actively expressed[19-21]. This mechanism may also help to reduce the sequestration of neutrophils in the pancreas and the associated OFR generation, thus effectively attenuating pancreatic damage.
In conclusion, overproduction of OFRs takes place in early SAP, and is a significant factor for aggravating pathogenetic condition. RESV can ameliorate pathological lesions in the pancreas by lowering pancreatic OFRs and reducing pancreatic tissue infiltration of neutrophils. It may have certain therapeutical effects on acute pancreatitis.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Wu M
| 1. | Kollár P, Hotolová H. [Biological effects of resveratrol and other constituents of wine]. Ceska Slov Farm. 2003;52:272-281. [PubMed] |
| 2. | Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 376] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Jang DS, Kang BS, Ryu SY, Chang IM, Min KR, Kim Y. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem Pharmacol. 1999;57:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Docherty JJ, Fu MM, Stiffler BS, Limperos RJ, Pokabla CM, DeLucia AL. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 1999;43:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Cheong H, Ryu SY, Kim KM. Anti-allergic action of resveratrol and related hydroxystilbenes. Planta Med. 1999;65:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Fontecave M, Lepoivre M, Elleingand E, Gerez C, Guittet O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998;421:277-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 272] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3633] [Cited by in RCA: 3391] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 8. | Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 496] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 9. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 674] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Bhatia M, Brady M, Zagorski J, Christmas SE, Campbell F, Neoptolemos JP, Slavin J. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Sanfey H, Bulkley GB, Cameron JL. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984;200:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Kishimoto W, Nakao A, Nakano M, Takahashi A, Inaba H, Takagi H. Detection of superoxide free radicals in rats with acute pancreatitis. Pancreas. 1995;11:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Demols A, Van Laethem JL, Quertinmont E, Legros F, Louis H, Le Moine O, Devière J. N-acetylcysteine decreases severity of acute pancreatitis in mice. Pancreas. 2000;20:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Rau B, Poch B, Gansauge F, Bauer A, Nüssler AK, Nevalainen T, Schoenberg MH, Beger HG. Pathophysiologic role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage. Ann Surg. 2000;231:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Lasztity N, Hamvas J, Biró L, Németh E, Marosvölgyi T, Decsi T, Pap A, Antal M. Effect of enterally administered n-3 polyunsaturated fatty acids in acute pancreatitis--a prospective randomized clinical trial. Clin Nutr. 2005;24:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Reinheckel T, Nedelev B, Prause J, Augustin W, Schulz HU, Lippert H, Halangk W. Occurrence of oxidatively modified proteins: an early event in experimental acute pancreatitis. Free Radic Biol Med. 1998;24:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Poch B, Gansauge F, Rau B, Wittel U, Gansauge S, Nüssler AK, Schoenberg M, Beger HG. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: mediators of local destruction and activators of inflammation. FEBS Lett. 1999;461:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Wisner J, Green D, Ferrell L, Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988;29:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 369] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 20. | Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509-6519. [PubMed] |
| 21. | Holmes-McNary M, Baldwin AS. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477-3483. [PubMed] |