EPIDEMIOLOGY
In recent years, the true prevalence of IBS has been documented in many parts of the world. What is truly remarkable is how common IBS is, no matter where you look! It is absolutely clear, for example, that IBS is a common disorder, not only in North America and Western Europe but throughout Asia and Latin America and even in parts of Africa[1]. However, caution needs to be exerted in the interpretation of such studies. Typically, community or hospital-based surveys of IBS prevalence have utilized some iteration of Rome or Manning criteria as their diagnostic instrument; whether these diagnostic tools, developed in the West, are equally valid in emerging nations, where confusion with symptoms related to chronic parasitic infestations, for example, may be an issue. Clearly, we have much to learn from the epidemiology and natural history of IBS or IBS-like symptoms in this context.
DIAGNOSIS
Diagnostic confusion has also emerged as an issue in the West. Here, still the debate continues regarding potential overlap between IBS, IBD and celiac sprue[2]. Do reported instances of celiac sprue among patients with “typical” IBS, or the occurrence of IBS-type symptoms among IBD patients in apparent remission, reflect a true association between these disorders and, thereby, the effects of low grade inflammation on enteric nerve and muscle function, or does such apparent overlap simply serve to emphasize the non-specificity of many gastrointestinal symptoms Explaining it simply, the gut has a limited symptomatic repertoire which may not allow us to differentiate between those complaints which are consequent upon continuing (but otherwise undetected) inflammation in IBD or in the non-compliant celiac and those which arise from a functional disorder, per se. Progress in this contentious area must await readily applicable measures of disease activity which are sufficiently sensitive and accurate to provide a true definition of remission.
In the meantime, how should the clinician interpret these dilemmas It is evident, that the majority of celiacs now present later in life and usually with vague and non-specific gastrointestinal symptomatology; celiac disease must, therefore, be considered in all new IBS patients, especially in areas of high prevalence and regardless of the nature of presenting symptoms[3].
ASSOCIATED DISEASES
Over the years, IBS has been associated with a wide variety of intestinal and extra-intestinal symptoms and syndromes. Recent community surveys have confirmed how frequently IBS, functional dyspepsia (FD) and gastroesophageal reflux disease (and non-erosive reflux disease (NERD), in particular) overlap; a phenomenon that may complicate clinical trials as well as diagnostic and therapeutic strategies. My own belief is that we should be “lumpers” and not “splitters” here; I contend that efforts to separate IBS from FD and NERD are clinically unrealistic and unhelpful. IBS has also been associated with a variety of psychological disorders; here, in contrast, the evidence for a true association is less firm, more recent analyses suggest that the occurrence of such symptomatology in IBS is largely the preserve of those who seek further referral alone and is not a feature of IBS in the community. Psychopathology should be viewed, therefore, not as a fundamental prerequisite for the development of IBS, but, rather, as a co-factor which, if present, will modify the individual’s response to IBS symptomatology. IBS patients commonly complain of fatigue and tiredness; these appear to be real entities in IBS, yet have been scarcely acknowledged in the assessment of IBS activity or response to therapy. Urinary and gynecological symptoms are also common; the basis for these associations is less clear. Aware of the prominence of smooth muscle hyper-reactivity in both conditions, parallels have been drawn between IBS and asthma; whether these conditions are linked remains to be defined.
PATHOPHYSIOLOGY
Genetic factors
While IBS patients commonly give a positive family history, the relative roles of “nature” and “nurture” in this intra-familial aggregation of functional disorders have received little attention. For example, in a recent community survey almost 20% of IBS sufferers reported abdominal symptoms in a first degree relative; a relative risk of 2.5[4]. Whether this association reflects reporting bias, shared environmental factors or a true genetic basis has been addressed in two recent twin studies which both identified a genetic component to IBS[5,6]. This is not the whole answer by any means; thus in the study by Levy and colleagues, while the concordance for IBS was twice as high in monozygotic than in dizygotic twins (15.2% vs 6.7%), a history of IBS in a parent was a more potent predictor of IBS in a twin than was the presence of IBS in the other twin[6]. These findings suggest a relatively minor role for genetic factors in the basic pathogenesis of IBS. Genetic factors may, however, influence disease expression and therapeutic response, as evidenced by recent studies of G-protein subunit, IL-10, CCK-1 receptor, alpha 2 adrenoceptor and serotonin transporter genotypes among IBS patients[7-11]. These are complex studies but may pave the way for real progress in our understanding of the true diversity of IBS [12].
Gastrointestinal motor dysfunction
Dysmotility has long been considered a major factor in the pathophysiology of IBS, as indicated by the use, in the past, of such terms as the “spastic colon” to describe what is now referred to as IBS. Accordingly, it was suggested that gut spasm or other abnormal contractile activities led to the development of symptoms in IBS. There are, indeed, several reports of abnormal motor patterns in many parts of the gastrointestinal tract in IBS. The specificity of many of these abnormalities for IBS is, however, unclear[13]. In contrast, and of particular interest, are very recent observations on the handling of gas by the intestine in IBS[14,15]. Whereas gas infused into the small intestine was rapidly evacuated through the gut in normal volunteers, a similar infusion resulted in gas retention, symptoms and an increase in abdominal girth in IBS patients[14]; all reversible by administration of a prokinetic agent[15]. Distension, often the most distressing “gas”-related symptom in IBS, has, until recently, been assumed to represent a disturbance of perception, as apparently objective tests of abdominal volume failed to detect any increase in IBS[16]. This assumption has now been questioned[17] and it may well come to pass that more detailed studies of changes in distension over time[18] may detect significant diurnal variations in girth in IBS. While the balance of evidence suggests that intestinal gas production is not abnormal in IBS, one can visualize how relatively local changes in the gas content could lead to symptoms, given the aforementioned intrinsic abnormality of gas transport[19] and the hypersensitivity to intraluminal gas that are known to occur in IBS[20].
Visceral hypersensitivity and hyperalgesia
Recently, there has been considerable interest in these phenomena, not only in IBS, but also in functional disorders, in general[7]. The phenomenon of visceral hypersensitivity, to distention and other intra-luminal stimuli, appears to be common in patients with non-cardiac pain, FD and the irritable bowel, alike. Recently, it has been suggested that visceral hyperalgesia, the phenomenon whereby stimuli normally not experienced as painful become so, is highly specific for IBS[21]. Visceral hypersensitivity, visceral hyperalgesia and viscero-somatic referral (the phenomenon whereby stimuli are referred over wide areas) have, indeed, been confirmed, in IBS, in more recent studies, using a variety of methodologies and under controlled experimental conditions[22]. While visceral hyperalgesia has been postulated as being highly specific for IBS, it alone or in association with other manifestations of hypersensitivity cannot explain all of IBS; even the most celebrated enthusiasts for the sensory hypothesis concede that sensation is normal in some patients.
There are several possible anatomical locations for sensory abnormalities in IBS, ranging from sensory receptors on the gut wall, primary sensory afferent neurons, to the spinal cord and the brain itself. Research in this area in man is notoriously difficult; however, advances in functional brain imaging provided by such techniques as cerebral evoked potentials (CEP), positron emission tomography (PET), magnetoencephalography (MEG) and functional magnetic resonance imaging (fMRI), have provided insights into the brain’s response to visceral stimuli. These and other studies have advanced the concept of an abnormal (or hypervigilant) central nervous system (CNS), in IBS, which records an exaggerated, inappropriate or aberrant perception of visceral events[23]. Other pieces of evidence support this concept. These include the conscious perception, by IBS patients, of intestinal motor events which are usually sub-conscious and evidence of abnormal psycho-neuro-hormonal responses, often implicating an abnormal hypothalamico-pituitary axis (HPA).
Motility and sensation may not be the most fundamental causes of IBS; it is clear, however, that these phenomena play a significant role in symptom generation.
Infection, inflammation, immunity and IBS
It may come as a real surprise to many to hear that infection and inflammation are now seen as potential factors in the etiology of IBS. With regard to infection, we are now beginning to see the real data to directly support the concept of post-infective or post-dysenteric IBS.
Infection and IBS: First reported by McKendrick and Read[24], the occurrence of IBS following bacteriologically-confirmed gastroenteritis has now been documented in several studies[25-30]. The risk of developing IBS following an episode of gastroenteritis is in the order of 4%-23%, with females, those with a more severe initial illness and pre-morbid psychopathology being most at risk[25,26,28,30]. One of these studies went on to establish a direct link between prior exposure to an infectious agent, persisting low-grade inflammation and IBS[28]. In this study, an increase in the number of chronic inflammatory cells in the rectal mucosa was seen only among those exposed patients who had developed IBS. Others have demonstrated a persisting increase in rectal mucosal enteroendocrine cells, T lymphocytes and gut permeability in patients with post-dysenteric IBS[29,30]. Post-infectious IBS may explain only a minority of cases of IBS but does represent a clear link between exposure to an environmental agent, inflammation and IBS, in predisposed individuals[31].
Inflammation and IBS: Direct and compelling evidence was first provided by Chadwick and colleagues for a role of inflammation in IBS, in general. They evaluated 77 IBS patients of whom 55% would be considered as diarrhea predominant; none had a confirmed infectious origin for their IBS[32]. All had colonic biopsies taken for conventional histology and immunohistology. Thirty-eight had normal histology, 31 demonstrated microscopic inflammation and 8 fulfilled the criteria for lymphocytic colitis. However, in the group with “normal” histology, immunohistology revealed increased intraepithelial lymphocytes as well as an increase in CD3+ and CD25+ cells in the lamina propria; all, therefore, showed evidence of immune activation. These features were even more evident in the microscopic inflammation group who, in addition, revealed increased neutrophils, mast cells and natural killer cells. All of these aforementioned immunopathological abnormalities were most evident in the lymphocytic colitis group who, alone, also demonstrated HLA-DR staining in crypts and increased CD8+ cells in the lamina propria. Interestingly, taking the group of IBS patients as a whole, CD3+ cell number was higher among those with diarrhea than among alternators or those with predominant constipation. In contrast, in the non-inflamed IBS group the presence of mast cells was a predictor of constipation. Surprisingly, given the aforementioned description of a direct relationship between symptoms and chronic inflammation among patients with post-infectious IBS, these authors did not find an association between either the nature of disease onset or disease duration and immunological findings. In an accompanying editorial, Collins suggested that the increased presence of CD25+ cells may have indicated “auto- or exogenous antigen challenge in these patients, and that the CD25+ cells are preventing the progression to a more florid inflammatory response”[33]. That IBS patients may be predisposed to an, albeit contained, inflammatory response to luminal triggers is also supported by the finding, of Gonsalkorale and colleagues, of a reduced frequency of the high-producer phenotype for the anti-inflammatory cytokine interleukin-10 (IL-10) among IBS patients[9]. A direct linkage between immune activation and symptoms has been provided by the work of Barbara and colleagues who demonstrated, not only an increased prevalence of mast cell degranulation in the colon in IBS, but also a direct correlation between the proximity of mast cells to neuronal elements and pain severity[34].
While the inflammatory hypothesis in IBS is in its infancy, there is already some evidence for the extension of the inflammatory process beyond the confines of the mucosal compartment. Tornblom and colleagues addressed this issue in ten patients with severe IBS by examining full-thickness jejunal biopsies obtained at laparoscopy[35]. In nine, they found low-grade infiltration of lymphocytes in the myenteric plexus; four of these had an associated increase in intraepithelial lymphocytes and six demonstrated evidence of neuronal degeneration. Nine patients had longitudinal muscle hypertrophy and seven had abnormalities in the number and size of interstitial cells of Cajal. Interestingly, three of their patients reported an acute onset of their IBS; in two, possibly precipitated by gastroenteritis. The finding of intraepithelial lymphocytosis is consistent with the reports of Chadwick and colleagues[32], in the colon and of Wahnschaffe and colleagues, in the duodenum[36]. Most recently, in a group of 78 unselected IBS patients, we demonstrated, in peripheral blood mononuclear cells, an alteration in the ratio between the cytokines IL10 and IL12 which became skewed towards a Th1, pro-inflammatory profile[37].
With regard to the pathophysiology of the mucosal inflammatory changes, Spiller proposed that these changes could represent a response to an initial bacterial infection among individuals who are rendered susceptible by a relative deficiency of anti-inflammatory cytokines[38]. Alternately, could this low-grade inflammation represent either an abnormal reaction to the normal flora or a contained response to qualitative or quantitative changes in the intrinsic flora Whether IBS is accompanied by quantitative or qualitative changes in the bacterial flora of the small or large intestine remains a contentious issue; while some have described bacterial overgrowth in the small intestine[39,40] and qualitative alterations in the fecal flora[41,43] and increased bacterial fermentation[44], in IBS, others have failed to replicate these findings[45]. The description of efficacy for certain probiotics, and bifidobacterium, in particular, in IBS[37] could also support a role of gut flora-mucosal interaction in IBS[46]. Bacterial overgrowth could also explain some of the proposed overlap between IBS and celiac sprue[47].
MANAGEMENT
Many IBS patients relate the onset of symptoms to intake of food and often incriminate specific food items. However, the role of food intolerance or food allergy in IBS has remained undefined. While most would agree that there is scant evidence for classical food allergy in IBS, Whorwell and colleagues suggest that testing for food intolerance, utilizing IgG antibodies, can lead to a successful dietary modification regime[48].
In recent years, much interest has been generated by serotonin and the potential role of serotonergic drugs in IBS[49]. Tegaserod, a 5HT4 agonist, is effective in the therapy of female patients with constipation-predominant IBS and has demonstrated efficacy against some previously “resistant” symptoms, such as bloating[50,51]. Alosetron, a 5HT3 agonist, is effective in females with diarrhea-predominant IBS, but its prescription is now limited due to reports on ischemic colitis[52]. Cilansetron, a 5HT3 agonist, is effective in both males and females with diarrhea-predominant IBS[53]; here the specter of ischemic colitis has again become an issue with regulators, in the US. Indeed, ischemic colitis has become an issue for all of these agents, though it appears that many reports of association probably reflect diagnostic confusion ab initio between IBS and ischemic colitis rather than an effect of serotonergic agents, per se[54].
Given the explosion that has occurred in our understanding of the enteric nervous system, and of the pathways that link it to the CNS, it should come as no surprise that many agonists and antagonists of other putative neurotransmitters and neuromodulators are under study in IBS and related disorders.
Given the potential role of infection and inflammation in at least some instances of IBS, efforts have been made to address this aspect of pathophysiology in IBS. In this regard, a probiotic, bifidobacterium infantis has proved to be a very successful agent in unselected IBS patients[37]. Clearly, this is an area of increasing interest.
CONCLUSIONS
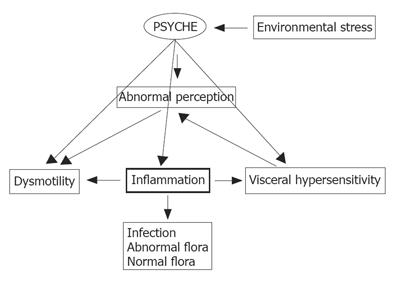
Our understanding of IBS has come a long way. This is a global disease associated with significant symptoms and impairments in personal and social functioning for afflicted individuals. Advances in our understanding of gut flora-mucosal interactions, the enteric nervous system and the brain-gut axis have led to substantial progress in the pathogenesis of symptoms in IBS and have provided some hints towards the basic etiology of this disorder, in some subpopulations, at the very least. We look forward to a time when therapy will be addressed to pathophysiology and, perhaps, even to primary etiology. In the meantime, as illustrated in Figure 1, I would suggest that a model based on a primary role for intestinal inflammation serves to integrate the various strands which contribute to the presentation of IBS.
Figure 1 Interactions between the gut, the brain and the external and internal environments in IBS; the Iinflammation hypothesis (a personal view).
Bacterial or viral infection, a disturbed flora or an abnormal response to a normal flora leads to mucosal Iinflammation which in turn can disrupt motility and augment visceral sensation. Centrally, perception is abnormal, thereby, contributing to symptom development. Central output can in turn influence motor events in the periphery. While not central to causation, psychological factors, either spontaneously or in response to environmental stressors, can influence motor and sensory events and immune activity in the gut.