Published online Mar 7, 2005. doi: 10.3748/wjg.v11.i9.1356
Revised: August 15, 2004
Accepted: October 5, 2004
Published online: March 7, 2005
AIM: In patients with liver cirrhosis, hypoalbuminemia causes edema and ascites, and a reduction in the quality of life. Since musculature is catabolized to supply amino acids for albumin synthesis in malnutritional cirrhotic patients, muscular volume is hypothesized to play an important role in albumin production. Therefore, we investigated the correlation between serum albumin levels and the fat-free mass (FFM) in cirrhotic patients.
METHODS: Fifty-seven patients (26 males and 31 females) with compensated liver cirrhosis were evaluated. Patients with edema or ascites were excluded from the study. Healthy volunteers (n = 104; 48 males and 56 females) were also evaluated as controls. FFM was measured using 5-500 kHz multifrequency bioelectric impedance analysis. To minimize the difference in FFM distribution between males and females, we introduced a new marker, relative FFM (rFFM), which represents the ratio of FFM in a patient relative to that in a volunteer of the same height. Following FFM measurement, the serum albumin levels of patients were assayed monthly.
RESULTS: In patients with active cirrhosis (alanine aminotransaminase [ALT] >50 U/L), both albumin (the difference between maximum and minimum levels) and the standard deviation of albumin levels (SD-albumin) during the observation period showed a significant correlation with rFFM. Multiple linear regression analysis using variables such as rFFM, platelet number, and serum cholesterol levels, choline esterase, albumin, bilirubin, and ALT revealed that rFFM and ALT were significant and independent factors that influenced albumin or SD-albumin in cirrhotic patients.
CONCLUSION: Our results indicate that cirrhotic patients with high rFFM showed less of a decrease in albumin levels, and that the muscle volume is one of the most important factors for maintaining serum albumins level in active cirrhosis. Exercise and protein-rich nutrition at the early stage of liver cirrhosis may be advisable for maintaining or increasing muscular volume.
- Citation: Kotoh K, Nakamuta M, Fukushima M, Matsuzaki C, Enjoji M, Sakai H, Nawata H. High relative fat-free mass is important for maintaining serum albumin levels in patients with compensated liver cirrhosis. World J Gastroenterol 2005; 11(9): 1356-1360
- URL: https://www.wjgnet.com/1007-9327/full/v11/i9/1356.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i9.1356
In earlier reports, several factors were established for predicting the prognosis of patients with liver cirrhosis, such as ascites, liver volume, encephalopathy, esophageal varices, serum albumin, serum bilirubin and clotting factors[1-5]. Among these factors, serum albumin level was commonly regarded as the most reliable. The usefulness of utilizing hepatic protein synthesis as a parameter for predicting the prognosis of cirrhotic patients is supported by a series of reports, which showed that a medication with branched amino acid elevated the serum levels of albumin and improved the prognosis[6-8]. Besides predicting survival, maintaining high albumin levels is clinically important for patients with liver cirrhosis, because decreased serum albumin levels cause ascites and edema, which lower the quality of life.
With respect to protein metabolism in cirrhotic patients, it should be emphasized that musculature plays an important role as an amino-acid pool. In healthy controls, the amino acid pool is distributed in the musculature (80%), liver (15%) and plasma (5%)[9]. In cirrhotic patients, starvation readily induces muscle protein catabolism because there is relatively little glycogen stored in the cirrhotic liver. Therefore, in the assessment of nutritional status in cirrhotic patients, the evaluation of muscle protein stores is important.
Bedrest is commonly prescribed for the management of patients with liver cirrhosis. However, it is obvious that a bedrest results in muscle volume reduction, which might reduce the serum levels of albumin. Therefore, it seems reasonable to postulate that cirrhotic patients with high muscle volume would be more resistant to reductions in serum albumin associated with the progression of liver damage. In order to confirm this hypothesis, we examined the relationship between serum albumin levels and body composition in cirrhotic patients.
A number of techniques are available for measuring body compartments, such as dual energy X-ray absorptiometry (DEXA), total body potassium measured by whole-body potassium-40 counting, total body water measured by isotope dilution, in vivo neutron activation analysis (IVNAA), and bioelectric impedance analysis (BIA). Those techniques, except BIA, associated with high cost and labor-intensive methods. Although BIA has been used to estimate fat-free mass (FFM) of cirrhotic patients at bedside, the validity of the measurements obtained with the single-frequency method has been questioned. Borghi (1996) showed that multifrequency BIA might yield valid body composition data for cirrhotic patients without ascites[10]. In the present study, we used multifrequency BIA for measuring FFM and we limited the study population to cirrhotic patients without ascites or edema.
Fifty-seven patients with compensated liver cirrhosis, who were outpatients of our department from April 2000 to March 2002, were evaluated (Table 1). They consisted of 26 males and 31 females, and ranged in age from 27 to 83 years. Fifty-two (92%) and five (8%) patients were classified as Child A and Child B without edema and ascites respectively. Fifty (88%) and 7 (12%) cases were caused by hepatitis C virus and hepatitis B virus, respectively. There was no significant difference in the basic characteristics between males and females (Table 1). Patients with edema were excluded from this study. Ultrasonography (US) was done to confirm that the evaluated patients did not have ascites. The patients were diagnosed with liver cirrhosis based on the results of liver biopsy and/or imaging studies (computed tomography and USA). One hundred and four volunteers (48 males and 56 females) were also evaluated as control.
| Variables | Male | Female | All |
| n | 26 | 31 | 57 |
| Age (yr) | 62.0±11.4 | 66.0±10.0 | 64.2±10.8 |
| Etiology (HCV/HBV/Alcohol/PBC) | 23/2/1/0 | 27/2/1/1 | 50/4/2/1 |
| Child-Pugh (A/B/C) | 23/3/0 | 29/2/0 | 52/5/0 |
| BMI | 23.7±2.2 | 22.0±2.7 | 22.8±2.6 |
| Albumin (g/dL) | 3.38±0.49 | 3.57±0.47 | 3.48±0.48 |
| Bilirubin (mg/dL) | 1.31±0.64 | 1.09±0.49 | 1.19±0.57 |
| AST (U/L) | 87.2±71.7 | 76.0±55.1 | 81.1±62.9 |
| ALT (U/L) | 77.7±72.3 | 64.9±70.1 | 70.7±70.8 |
| Cholesterol (mg/dL) | 155.3±38.2 | 156.3±27.3 | 155.9±32.3 |
| ChE (mg/dL) | 83.0±32.2 | 92.0±38.9 | 88.0±36.0 |
| Platelet (×104/L) | 9.1±3.8 | 10.9±6.5 | 10.1±5.5 |
For all patients, 5-500 kHz multifrequency BIA was performed using InBody 3.0 (Exercise Physiology USA and Biospace Co., Ltd.)[11]. On the same day, they underwent general laboratory examination for albumin, bilirubin, cholesterol, aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), and blood platelet count. The patients and control volunteers fasted overnight prior to the laboratory examinations. FFM was calculated as described previously[10,11]. After measurement of FFM, serum biochemistry assays were performed every month on an outpatient basis. The average observation period was 15.4 mo, ranging from 12 to 24 mo.
The results of laboratory data are shown as mean±SD. Differences in the average and dispersion of FFM and rFFM between males and females were confirmed by t-test and f-test. Stepwise multivariate linear regression analysis was performed to confirm the significant predictive factors for albumin stability using variables such as rFFM, albumin, bilirubin, cholesterol, ALT, and blood platelet count.
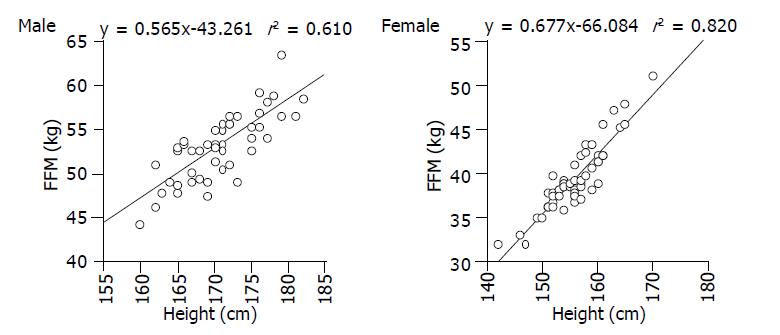
In order to evaluate the relationship between FFM and height, 48 male and 56 female volunteers, whose body mass index (BMI) ranged from 20 to 24, underwent multifrequency BMI. As shown in Figure 1, there was a good correlation between FFM and height for males and females, and the standard FFM could be calculated as below.Male: standard FFM (kg) = 0.565×height (cm) - 43.261; Female: standard FFM (kg) = 0.677×height (cm) - 66.084.
According to the regression formula above, expected FFM was calculated using the height for each patient. Then relative FFM (rFFM) was then calculated as follows. rFFM (%) = (measured FFM/standard FFM) ×100. In short, rFFM represents the ratio between the FFM of a patient and that of a normal control with an average structure and the same height as the patient.
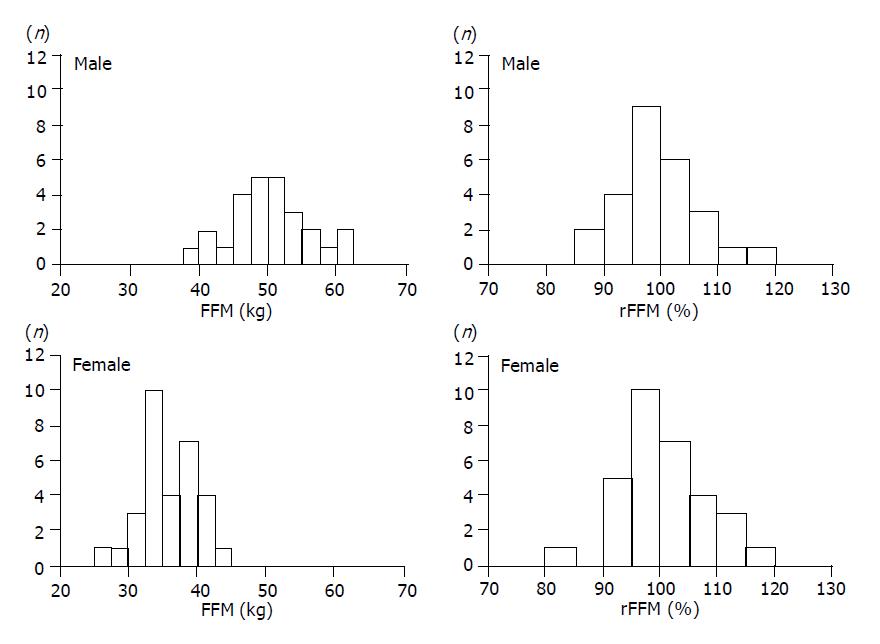
The left columns in Figure 2 show the FFM distributions of cirrhotic patients, and there was a significant difference in dispersion between males and females (P<0.001). In calculating rFFM for the patients (right columns of Figure 2), the dispersion did not differ significantly between males and females. Therefore, it was possible to compare rFFM of patients regardless of their sex differences.
Serum albumin levels were measured for all patients every month. Using the monthly results, standard deviation was calculated for all patients (SD-albumin). The difference between the maximum and minimum results during the entire observation period was also calculated (albumin). Both SD-albumin and -albumin were considered to be indications of the stability of hepatic synthetic function.
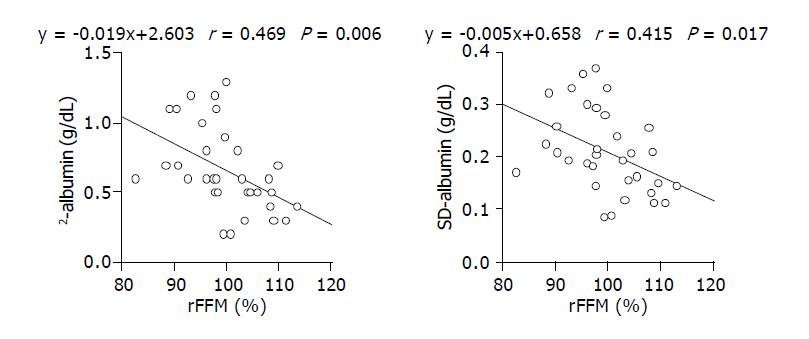
Comparison of rFFM and SD-albumin, or rFFM and -albumin for all patients, showed no significant correlations. However, when the analysis was limited to patients with average ALT levels over 50 U/L, we found significant correlations between rFFM and SD-albumin, and between rFFM and -albumin (Figure 3).
It is reasonable that rFFM correlates significantly with SD-albumin and -albumin in patients with high ALT levels, since the demand for amino acids would be increased under conditions in which hepatocytes are regenerating. However, other factors, such as the hepatic functional reserve or nutritional status at the time of rFFM measurement, might also influence SD-albumin and -albumin.
To investigate this possibility, we performed stepwise regression analysis using variables such as rFFM, platelet number, and the serum levels of cholesterol, chorine esterase, albumin, bilirubin and ALT. This analysis revealed that rFFM and ALT were independent predictors for both-albumin and SD-albumin (Table 2, Table 3).
| vs -Albumin | |||||
| Univariate analysis | Multivariate analysis | ||||
| R2 | F | P | F | P | |
| rFFM | 0.094267 | 5.6202 | 0.0214 | 6.0402 | 0.0173 |
| Albumin | 0.016847 | 0.9254 | 0.3404 | - | - |
| Bilirubin | 0.017005 | 0.9342 | 0.3381 | - | - |
| ALT | 0.08064 | 4.7365 | 0.0339 | 5.0355 | 0.029 |
| Cholesterol | 0.009282 | 0.4965 | 0.4841 | - | - |
| Platelet | 0.021799 | 1.1811 | 0.2821 | - | - |
| vs SD-Albumin | |||||
| Univariate analysis | Multivariate analysis | ||||
| R2 | F | P | F | P | |
| rFFM | 0.06456 | 3.7268 | 0.0488 | 3.8602 | 0.0447 |
| Albumin | 0.02243 | 1.239 | 0.2706 | - | - |
| Bilirubin | 0.003762 | 0.2039 | 0.6534 | - | - |
| ALT | 0.072818 | 4.241 | 0.0443 | 4.3666 | 0.0415 |
| Cholesterol | 0.005361 | 0.2857 | 0.5952 | - | - |
| Platelet | 0.02186 | 1.1845 | 0.2814 | - | - |
It has been widely accepted that the determination of body composition is useful for evaluating nutritional status. However, traditional methods, such as skinfold measurement, are easy to perform but lack accuracy and reproducibility. On the other hand, other methods such as tracer dilution, neutron activation analysis and DEXA require sophisticated equipments and skilled technique. In recent years, BIA has emerged as a simple and reproducible method that can be used for the evaluation of FFM. Several reports have shown that the results of BMI correlate well with those of other methods[12,13].
Regardless of the method used for the analysis of body composition, sex differences have been a substantial obstacle. The results of measurements in males and females cannot be readily compared since their average body composition values differ significantly. To solve the problem, we applied a new factor, rFFM (= [measured FFM/standard FFM]×100%). Using this new variable, we could compare the FFM results of all patients, regardless of their sex. Although we used the formula derived from the correlation between the FFM and height of the controls in calculating rFFM, the average body compositions are known to differ by race[14,15]. Therefore, the controls for standard curves should be prepared for each individual study.
Our results revealed that rFFM correlated with -albumin and SD-albumin in patients with high ALT levels, which indicated that muscle volumes would be one of the most important factors for maintaining the serum albumin level in active cirrhosis. This result seems reasonable since muscle protein serves as an amino acid pool. It should be noted that the stability of serum albumin levels is important not only for predicting the prognosis of patients with liver cirrhosis, but also to ensure a high quality of life. Serum albumin accounts for the colloid osmotic pressure of plasma; therefore hypoalbuminemia causes ascites, edema, and a reduction of circulating plasma volume, which would exacerbate hepatic failure.
In order to maintain or improve muscle volume, two interventions should be considered namely, amino acid supplementation and exercise. Cirrhotic patients often suffer from negative energy balance even at an early stage of the disease, characterized predominantly by protein deficiency and it is well known that they also have reduced plasma branched-chain amino acid (BCAA). Recent studies demonstrated the efficacy of branched-chain amino acid supplementation in improving the hypoalbuminemia of cirrhotic patients[7,8]. In the present study, none of the patients had received BCAA-supplementation because decompensated patients were excluded. Theoretically, BCAA-supplementation could inhibit muscle catabolism and contribute to the maintenance of muscle volume.
Regarding the usefulness of exercise for cirrhotic patients, most reports have focused on decompensated patients with ascites. Salo et al[16], noted that moderate physical exercise caused a marked impairment in the renal function of patients with ascites as well as marked stimulation of vasoconstrictor systems, whereas Garcia-Pagan et al[17], indicated that moderate exercise increased portal pressure and might therefore increase the risk of variceal bleeding in patients with esophageal varices. Although the contribution of exercise to the prognosis of cirrhotic patients remains unclear, we believe that exercise at the compensated stage would be useful for maintaining muscle volume which could delay the emergence of symptoms such as ascites and edema that accompany the progression of cirrhosis. Once ascites emerge in a cirrhotic patient, abdominal fullness causes appetite loss, which induces further hypoalbuminemia and reduction of hepatic blood flow. To avoid such an injurious cycle, it is important to prevent hypoalbuminemia at the compensated stage. Taken together, the evidence suggests that the periodic evaluation of body composition using BAI, in addition to the physician’s recommendation for exercise, would be useful for improving the prognosis of compensated cirrhotic patients.
Assistant Editor Guo SY Edited by Gabbe M
| 1. | Tsuji Y, Koga S, Ibayashi H, Nose Y, Akazawa K. Prediction of the prognosis of liver cirrhosis in Japanese using Cox's proportional hazard model. Gastroenterol Jpn. 1987;22:599-606. [PubMed] |
| 2. | Zoli M, Cordiani MR, Marchesini G, Iervese T, Labate AM, Bonazzi C, Bianchi G, Pisi E. Prognostic indicators in compensated cirrhosis. Am J Gastroenterol. 1991;86:1508-1513. [PubMed] |
| 3. | Salerno F, Borroni G, Moser P, Badalamenti S, Cassarà L, Maggi A, Fusini M, Cesana B. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol. 1993;88:514-519. [PubMed] |
| 4. | Møller S, Bendtsen F, Christensen E, Henriksen JH. Prognostic variables in patients with cirrhosis and oesophageal varices without prior bleeding. J Hepatol. 1994;21:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Magliocchetti N, Torchio P, Corrao G, Aricò S, Favilli S. Prognostic factors for long-term survival in cirrhotic patients after the first episode of liver decompensation. Ital J Gastroenterol Hepatol. 1997;29:38-46. [PubMed] |
| 6. | Habu D, Nishiguchi S, Nakatani S, Kawamura E, Lee C, Enomoto M, Tamori A, Takeda T, Tanaka T, Shiomi S. Effect of oral supplementation with branched-chain amino acid granules on serum albumin level in the early stage of cirrhosis: a randomized pilot trial. Hepatol Res. 2003;25:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 8. | Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Kuntz E, Kuntz H. Biochemistry and functions of the liver in: Kuntz E, ed. Hepatology: Principles and practice. Berlin: Springer Verlag 2002; 25-62. |
| 10. | Borghi A, Bedogni G, Rocchi E, Severi S, Farina F, Battistini N. Multi-frequency bioelectric impedance measurements for predicting body water compartments in patients with non-ascitic liver cirrhosis. Br J Nutr. 1996;76:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Bedogni G, Malavolti M, Severi S, Poli M, Mussi C, Fantuzzi AL, Battistini N. Accuracy of an eight-point tactile-electrode impedance method in the assessment of total body water. Eur J Clin Nutr. 2002;56:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Wattanapenpaiboon N, Lukito W, Strauss BJ, Hsu-Hage BH, Wahlqvist ML, Stroud DB. Agreement of skinfold measurement and bioelectrical impedance analysis (BIA) methods with dual energy X-ray absorptiometry (DEXA) in estimating total body fat in Anglo-Celtic Australians. Int J Obes Relat Metab Disord. 1998;22:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Fogelholm M, van Marken Lichtenbelt W. Comparison of body composition methods: a literature analysis. Eur J Clin Nutr. 1997;51:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985). 2000;89:465-471. [PubMed] |
| 15. | Luke A, Rotimi CN, Adeyemo AA, Durazo-Arvizu RA, Prewitt TE, Moragne-Kayser L, Harders R, Cooper RS. Comparability of resting energy expenditure in Nigerians and U.S. blacks. Obes Res. 2000;8:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Saló J, Guevara M, Fernández-Esparrach G, Bataller R, Ginès A, Jimenez W, Ginès P, Rivera F, Arroyo V, Rodés J. Impairment of renal function during moderate physical exercise in cirrhotic patients with ascites: relationship with the activity of neurohormonal systems. Hepatology. 1997;25:1338-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | García-Pagàn JC, Santos C, Barberá JA, Luca A, Roca J, Rodriguez-Roisin R, Bosch J, Rodés J. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |