Published online Mar 7, 2005. doi: 10.3748/wjg.v11.i9.1345
Revised: August 18, 2004
Accepted: September 8, 2004
Published online: March 7, 2005
AIM: To investigate the differential phosphorylation and activation of p38 in hepatocytes by pro-apoptotic Transforming Growth Factor-β1 (TGF-β1), pro-survival factors Epidermal Growth Factor (EGF) and 12-O-tetradecanoylphorbol-13-acetate (TPA) and the potential mechanisms.
METHODS: The phosphorylation and activation of p38 were determined by immunoblotting. Apoptosis was analyzed by morphological staining and observation, FACS analysis of sub-G1 content and DNA fragmentation assay. To quantitatively determine caspase activation, caspase activity assay was performed in vitro.
RESULTS: TGF-β1-induced apoptosis was associated with the phosphorylation of p38, and SB202190, a specific inhibitor of p38, which was able to inhibit TGF-β1-induced caspase activation and apoptosis. TPA and EGF also blocked apoptosis induced by TGF-β1. Both of them induced the phosphorylation of p38. The results showed SB202190 had no effect on TGF-β1-induced phosphorylation of p38, but effectively inhibited both EGF and TPA-induced phosphorylation of p38.
CONCLUSION: Pro-apoptotic TGF-β1, anti-apoptotic TPA and EGF induce the phosphorylation of p38 through different mechanisms that can be distinguished by SB202190. The data suggest that TPA and EGF-induced p38 phosphorylation is through an autophosphorylation-dependent mechanism. Since p38 phosphorylation induced by TGF-β1 plays an important role in caspase activation and apoptosis, TPA and EGF-induced p38 phosphorylation may not be requisite for their anti-apoptotic function.
- Citation: Guo LX, Xie H. Differential phosphorylation of p38 induced by apoptotic and anti-apoptotic stimuli in murine hepatocytes. World J Gastroenterol 2005; 11(9): 1345-1350
- URL: https://www.wjgnet.com/1007-9327/full/v11/i9/1345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i9.1345
Apoptosis is a highly regulated cell suicide program characterized by several morphological and biochemical hallmarks, including membrane bleb, DNA fragmentation, accumulation of sub-G1 DNA content, and caspase-dependent proteolysis of a series of cellular substrates[1-3]. Apoptosis is important for balancing cell number during development and maintenance of the integrity and homeostasis of multicellular organism[4]. In liver, the fine-tuned apoptosis has been implicated in liver development and regeneration, and deregulation of apoptosis occuring in hepatocellular carcinomas[5].
TGF-β1 is the prototype of TGF-β cytokine family that has diverse effects on multiple physiological processes, including apoptosis, proliferation, and differentiation. After binding to its receptors, TGF-β is capable of activating SMAD transcriptional factors to regulate the expression of target genes[6]. TGF-β-induced apoptosis is one of the active fields in the investigation of TGF-β signaling. Either SMAD pathway or other pathways, including Daxx/JNK and TAK1/p38, can mediate TGF-β-induced apoptosis[7]. In the liver, TGF-β is a potent inducer of apoptosis for both hepatocytes and hepatoma cells[5,8].
Mitogen-activated protein kinase (MAPK) is a family of serine/threonine kinases composed of extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal kinase (JNK). p38 can be activated in response to various stimuli and has been implicated in the regulation of diverse physiological processes, including growth arrest, proliferation, differentiation, inflammation, and apoptosis[9]. The basic arrangement of the signaling cascade leading to MAPK activation consists of three kinases: a MAPK kinase kinase (MAPKKK) activates MAPK kinase (MAPKK), which in turn phosphorylates and activates MAPK[10]. It has been demonstrated that p38 is involved in TGF-β-induced apoptosis[11]; although it was also shown that p38 is associated with, but not required for TGF-β-induced apoptosis[12]. The mechanism utilized by TGF-β to activate p38 remains unclear. It was shown that TAK1 is able to act as an MAPKKK activated by TGF-β to phosphorylate and activate p38[13]. Furthermore, Dadd45b, which was regulated by SMAD, was also shown to be responsible for activating p38 in hepatocyte[14]. Recently, it was reported TGF-β is able to activate p38 through a receptor-dependent but SMAD-independent mechanism[15].
For a long time, phosphorylation of p38 on specific tyrosine and threonine residues by upstream MAPKK has been taken as the sole activation mechanism for p38. P38 inhibitors, including SB202190, have been widely used to investigate the activation and involvement of p38 in various basic physiological processes. Interestingly, several reports demonstrated that p38 inhibitors have no effect on both basal and stimuli-induced p38 phosphorylation, but indeed inhibit the kinase activity of p38[16,17]; on the other hand, these inhibitors inhibit phosphorylation of p38 in some cases[18]. These results imply that the intrinsic kinase activity of p38 is differentially involved in the phosphorylation of p38 under different conditions. Recently, a report demonstrated that there existed an auto-phosphorylation-dependent mechanism serving as an alternative pathway responsible for the phosphorylation and activation of p38[19].
In this study, we showed that the phosphorylation and activation of p38 is involved in TGF-β1-induced murine hepatocytes apoptosis. While EGF and TPA can inhibit apoptosis, these pro-survival factors also induce phosphorylation of p38. Our data showed p38 inhibitor SB202190 has differential effect on p38 phosphorylation induced by different stimuli: it has no effect on TGF-β1-induced p38 phosphorylation, while effectively inhibiting both EGF and TPA-induced p38 phosphorylation. These results indicate the auto-phosphorylation-dependent mechanism is responsible for pro-survival EGF and TPA-induced p38 phosphorylation, but not involved in pro-apoptotic TGF-β1-induced activation of p38.
Cell culture reagents were purchased from Life Technologies (Grand Island, NY). Acridine orange (AO)/ethidium bromide (EB) and propidium iodide (PI) were purchased from Sigma (St. Louis, MO). Nitrocellulose membrane was bought from Amersham Pharmacia Biotech (Buckinghamshire, UK). Super signal reagents were purchased from Pierce (Rockford, IL). SB202190 and horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody were bought from CALBIOCHEM (La Jolla, CA). Rabbit polyclonal antibodies against p38 and phospho-p38 were from Cell Signal Technology, Inc. (Beverly, MA).
AML-12 cells were grown in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium containing insulin (5 μg/mL), transferrin (5 μg/mL), selenium (5 μg/mL), dexamethasone (40 ng/mL), 100 units/mL penicillin and 100 μg/mL streptomycin and 10% fetal calf serum. Cell cultures were maintained at 37 °C in a humidified atmosphere of 50 mL/L CO2.
Cells were lysed in 10 mmol/L Tris (pH 7.4), 1 mmol/L EDTA, 0.5 mmol/L EGTA, 150 mmol/L NaCl, 1% Triton X-100, 50 mmol/L NaF, 10 mmol/L Na4P2O7·10H2O, 5 μg/mL aprotinin, 5 μg/mL leupeptin, and 1 mmol/L PMSF. Fifty milligrams of proteins were electrophoresed in SDS-polyacrylamide gel and transferred onto nitrocellulose membranes. The membranes were blocked with 5% milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) and subsequently incubated with primary antibody overnight at 4 °C. After washing with TBS-T for 30 min at room temperature, the membrane was further incubated with a horseradish peroxidase conjugated secondary antibody for 1 h followed by 1 h washing. Protein bands were visualized with super signal reagents.
Apoptotic rate was quantitatively determined by flow cytometry analysis. The percentage of cells with sub-G1 DNA content was taken as a measure of the apoptotic rate of the cell population. After the indicated treatment, cells were trypsinized and fixed with 70% ethanol overnight. Cells were then pelleted and RNA was removed by adding RNase (10 mg/mL) at 37 °C for 1 h. Finally, the cells were stained with propidium iodide and DNA content was analyzed by FACS (Becton-Dickinson FACSCalibur, the excitation wavelength is 488 nm).
DNA fragmentation of apoptotic cells was detected as described[3] with minor modifications. The cells were lysed on ice for 30 min in lysis buffer containing 10 mmol/L Tris-Cl pH 8.0, 25 mmol/L EDTA pH 8.0, and 0.25% Triton X-100. After centrifugation at 14000 g for 15 min, the supernatant was incubated with RNase at 37 °C for 1 h and then with proteinase K at 56 °C overnight. DNA were extracted sequentially with phenol, phenol : chloroform (1:1), and chloroform. The DNA in aqueous phase was precipitated and separated by 1.5% agarose gel electrophoresis, and then visualized and photographed under transmitted UV light.
Cell morphology was examined by staining cells with AO (1 μg/mL) and EB (1 μg/mL). Fluorescence was visualized immediately with a fluorescent microscope. The normal cells appear uniformly green; early apoptotic cells were stained green and contained bright green dots in the nuclei as a consequence of chromatin condensation and nuclear fragmentation; late apoptotic cells will be incorporated by ethidium bromide and therefore stained orange with condensed and often fragmented nuclei.
Cells were treated as indicated and collected by trypsinization. Cells were resuspended in lysis buffer (50 mmol/L HEPES, pH 7.4, 100 mmol/L NaCl, 0.1% CHAPS, 1 mmol/L DTT, 0.1 mmol/L EDTA) for 15 min on ice and centrifuged at 14000 g at 4 °C. The supernatants were collected as the sample for caspase activity detection. The assay was performed using a colorimetric substrate-based kit (Calbiochem) as the manufacturer’s instruction.
Results are presented as mean±SD for the number of experiments indicated. For statistical analysis, Student’s t test was performed. Differences were considered significant at a level of P<0.05.
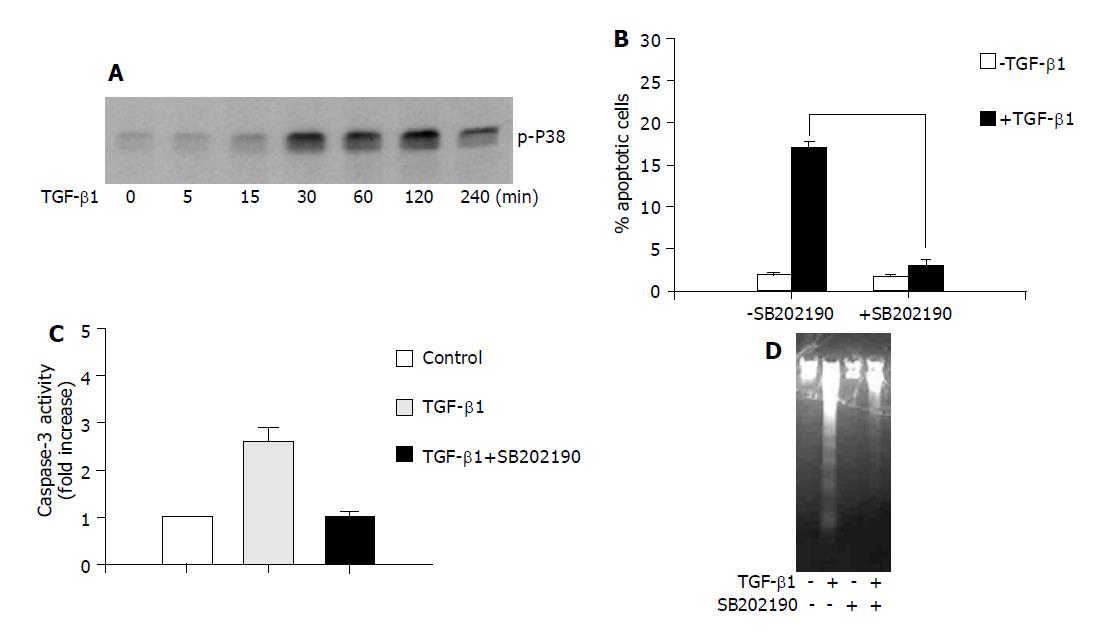
Previously, it was reported that p38 is phosphorylated and activated, likely through a SMAD-dependent mechanism, during TGF-β1-induced apoptosis[14]. As shown in Figure 1A, TGF-β1 induced rapid p38 activation as determined by increased phosphorylated p38 in AML-12 hepatocytes, which started at 30 min and decreased at 4 h after TGF-β1 administration. To address the role of p38 activation in apoptosis induction, we tested the effect of SB202190, the specific p38 inhibitor, on TGF-β1-induced apoptosis. It was shown in Figure 1B that SB202190 effectively inhibited TGF-β1-induced apoptosis as quantified by flow cytometry analysis of sub-G1 DNA content. These results suggest p38 activation by phosphorylation is critical for TGF-β1-induced apoptosis.
It is widely accepted that apoptosis is a cell-suicide program dependent on caspase activation, although caspase-independent cell death has been documented. Caspase activation has also been shown to be involved in TGF-β1-induced apoptosis[7]. To further explore the mechanism of the anti-apoptotic action of p38 inhibition, we performed a caspase-3, one of the executioner caspases, activity assay to examine the caspase-3 activity in the presence or absence of SB202190 in response to TGF-β1. As shown in Figure 1C, TGF-β1-induced apoptosis was associated with the activation of caspase-3, and SB202190 significantly inhibited TGF-β1-induced caspase-3 activity. Consistent with this result, the DNA fragmentation induced by TGF-β1 was also significantly blocked in the presence of SB202190 (Figure 1D). These results suggest that caspase activation is responsible for TGF-β1-induced apoptosis and p38 inhibition by SB202190 inhibits apoptosis by blocking caspase activation.
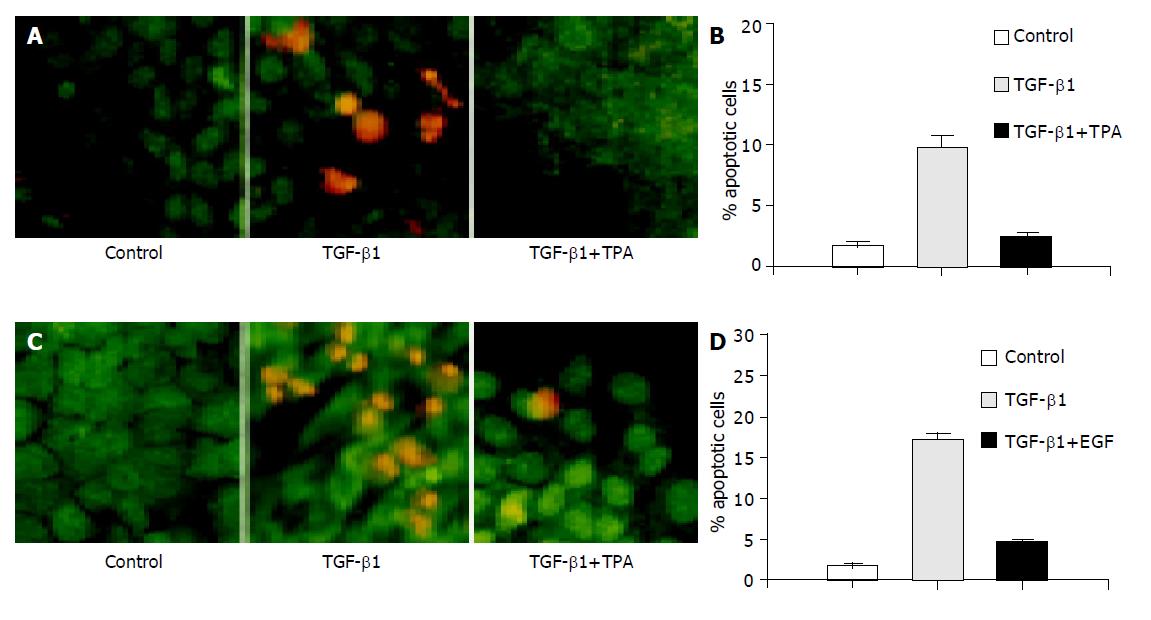
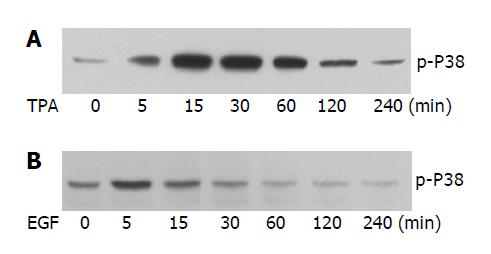
It is regarded that apoptosis can be blocked through different mechanisms, including direct antagonism of pro-apoptotic signaling elicited by apoptotic stimuli and activation of pro-survival signaling. It has been reported that TPA and EGF are involved in the regulation of apoptosis induced by different external stimuli[20,21]. To further understand the anti-apoptotic mechanism of TPA and EGF, we also tested their effect on TGF-β1-induced apoptosis in AML-12 cells. As shown in Figure 2A, the presence of TPA completely abolished apoptosis induced by TGF-β1, as determined by both AO/EB staining of apoptotic cells (upper) and flow cytometry quantification of sub-G1 contents (bottom). Similarly, co-administration of the cells with EGF also significantly inhibited TGF-β1-induced apoptosis examined by either AO/EB staining (Figure 2B, upper) or flow cytometry analysis (Figure 2B, bottom). Considering the critical role of p38 phosphorylation and activation in TGF-β1-evoked apoptotic signaling, we subsequently determined the effect of TPA and EGF on the activation of p38. Interestingly, both TPA (Figure 3A) and EGF (Figure 3B) induced transient but rapid activation of p38, as determined by increase of phosphorylated p38. Furthermore, we also examined the effect of TPA and EGF on TGF-β1-induced p38 phosphorylation, but no significant effect was observed (data not shown). These results suggest that both TPA and EGF block TGF-β1-induced apoptosis in hepatocytes, likely through an indirect mechanism instead of direct antagonism of pro-apoptotic p38 signaling.
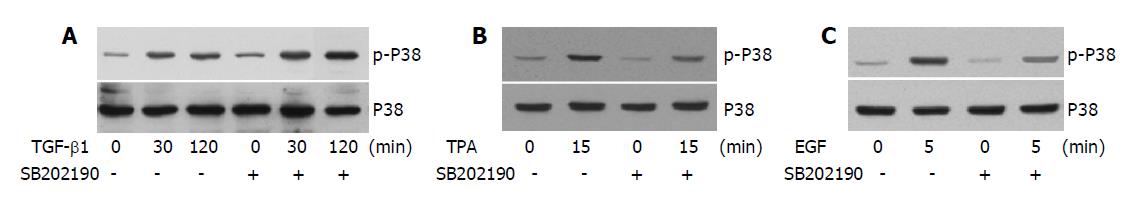
The fact that both pro-apoptotic TGF-β1 and anti-apoptotic TPA and EGF induce the phosphorylation of p38 promotes us to examine whether these factors share a common mechanism leading to this phenomenon. We tested the effect of SB202190 on the phosphorylation of p38 induced by different stimuli. Interestingly, it seemed SB202190 had little effect on TGF-β1-induced p38 phosphorylation (Figure 4A). On the contrary, both TPA and EGF-induced p38 phosphorylation can be effectively inhibited by SB202190 (Figures 4B, C respectively). These results suggested that the intrinsic kinase activity of p38 is necessary for TPA and EGF-induced p38 phosphorylation, but not required for TGF-β1-induced p38 phosphorylation that was likely catalyzed by an upstream kinase insensitive to SB202190. In conclusion, the results show that differential mechanisms are involved in TGF-β1, TPA and EGF-induced p38 phosphorylations.
Currently, the mechanism responsible for TGF-β-induced p38 activation and its potential role in apoptosis induction remains to be further clarified. It seems that the role of p38 activation in TGF-β-induced apoptosis is largely dependent on certain cellular context. On one hand, it was shown that p38 is involved in TGF-β-induced apoptosis[11]; on the other hand, it was shown that p38 activation by TGF-β in primary hepatocytes is associated with, but may not be required for apoptosis induction[12]. Here, consistent with a previous report[11], our data clearly show that TGF-β1 induces phosphorylation and activation of p38, and p38 activation plays a pivotal role in TGF-β1-induced apoptosis in AML-12 hepatocytes. Considering the fact that p38 inhibition by SB202190 significantly blocks TGF-β1-induced caspase activation and apoptosis, we postulated that p38 activation by TGF-β1 results in apoptosis through a mechanism involving the regulation of caspase activation.
Apoptosis is a highly organized program that is under the orchestrated balance of cellular pro-apoptotic and pro-survival signaling. Therefore, TGF-β1-induced apoptosis can also be blocked by either direct antagonism of pro-apoptotic signaling, for example inhibition of p38 activation in this study or activation of pro-survival signaling. EGF and TPA have already shown to be involved in the regulation of cell growth, survival, and apoptosis. Here, we found that both EGF and TPA significantly block TGF-β1-induced apoptosis in AML-12 hepatocytes. Interestingly, it seems that the anti-apoptotic effect of both factors only last for a relatively short period, and they are incapable of inhibiting apoptosis after long time, for example 48-h incubation with TGF-β1 (data not shown). We hypothesize that the anti-apoptotic effect of EGF and TPA is mainly due to the activation of pro-survival signaling rather than direct antagonism of TGF-β1-elicited apoptotic signaling. We therefore, examine the effect of both factors on the activation of p38. The results show both EGF and TPA can induce rapid phosphorylation of p38, although the phosphorylation is transient. Accordingly, the presence of both factors has no apparent effect on TGF-β1-induced phosphorylation of p38 (data not shown). These data support our hypothesis that the anti-apoptotic effect of EGF and TPA is not directly correlated with the antagonism of TGF-β1-induced pro-apoptotic p38 signaling. The results also suggest that the activation of p38 may not be required for the anti-apoptotic function of EGF and TPA.
The fact that both apoptotic TGF-β1 and pro-survival EGF, and TPA induce the activation of p38 promotes us to further determine the mechanisms responsible for p38 activation by these factors with distinct effect on cell death and survival. It is clear now that besides classical MAPKKK-MAPKK-p38 kinase cascade, a TAB1-mediated autopho-sphorylation-dependent mechanism is also able to be responsible for the activation of p38[19]. Therefore, the intrinsic kinase activity of p38 should play differential roles in the regulation of its phosphorylation under different conditions, for example in response to different external stimuli. Interestingly, our data show that SB202190 has no effect on TGF-β1-induced phosphorylation of p38, while completely inhibiting that induced by both EGF and TPA. The results suggest that different mechanisms are involved in the regulation of p38 activation induced by TGF-β1, EGF and TPA. While the intrinsic p38 kinase activity is critical for EGF and TPA-induced activation, it is not required for TGF-β1-induced p38 activation. It is thus logical to postulate that an upstream kinase insensitive to SB202190 is responsible for the activation of p38 by TGF-β1. This is consistent to the recent finding that TAK1 can function as a MAPKKK to transmit TGF-β signaling to activate p38[13,22]. Although both pro-apoptotic TGF-β1 and pro-survival EGF and TPA activate p38, their actual roles in apoptosis induction or anti-apoptotic action remain unclear. It will be interesting to further determine if p38 activation through different mechanisms affects its different downstream effectors leading to different cell fates, namely death or survival.
We thank Ya-Nan Yang for helpful discussion and technical assistance. We also thank Dr. Jian-Guo Song for encouragement and kind help.
Assistant Editor Guo SY Edited by Gabbe M
| 1. | Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5259] [Cited by in RCA: 5203] [Article Influence: 208.1] [Reference Citation Analysis (0)] |
| 2. | Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 985] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 3. | Yang Y, Zhao S, Song J. Caspase-dependent apoptosis and -independent poly(ADP-ribose) polymerase cleavage induced by transforming growth factor beta1. Int J Biochem Cell Biol. 2004;36:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109 Suppl:S97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 328] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2875] [Cited by in RCA: 2919] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 7. | Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 316] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Si XH, Yang LJ. Extraction and purification of TGFbeta and its effect on the induction of apoptosis of hepatocytes. World J Gastroenterol. 2001;7:527-531. [PubMed] |
| 9. | Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1244] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 10. | Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351 Pt 2:289-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Liao JH, Chen JS, Chai MQ, Zhao S, Song JG. The involvement of p38 MAPK in transforming growth factor beta1-induced apoptosis in murine hepatocytes. Cell Res. 2001;11:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Herrera B, Fernández M, Roncero C, Ventura JJ, Porras A, Valladares A, Benito M, Fabregat I. Activation of p38MAPK by TGF-beta in fetal rat hepatocytes requires radical oxygen production, but is dispensable for cell death. FEBS Lett. 2001;499:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J Biol Chem. 2000;275:17647-17652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ, Liebermann DA, Bottinger EP, Roberts AB. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem. 2003;278:43001-43007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Yu L, Hébert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749-3759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 571] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 16. | Waterman WH, Molski TF, Huang CK, Adams JL, Sha'afi RI. Tumour necrosis factor-alpha-induced phosphorylation and activation of cytosolic phospholipase A2 are abrogated by an inhibitor of the p38 mitogen-activated protein kinase cascade in human neutrophils. Biochem J. 1996;319:17-20. [PubMed] |
| 17. | Leonard M, Ryan MP, Watson AJ, Schramek H, Healy E. Role of MAP kinase pathways in mediating IL-6 production in human primary mesangial and proximal tubular cells. Kidney Int. 1999;56:1366-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Bordin S, Whitfield D. Cutting edge: proliferating fibroblasts respond to collagenous C1q with phosphorylation of p38 mitogen-activated protein kinase and apoptotic features. J Immunol. 2003;170:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 421] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Musallam L, Ethier C, Haddad PS, Bilodeau M. EGF mediates protection against Fas-induced apoptosis by depleting and oxidizing intracellular GSH stocks. J Cell Physiol. 2004;198:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Motyl T, Kasterka M, Grzelkowska K, Ostrowski J, Filipecki M, Malicka E, Pioszaj T. Phorbol ester (12-O-tetradecanoylphorbol 13-acetate) prevents ornithine decarboxylase inhibition and apoptosis and L1210 leukemic cells exposed to TGF-beta 1. Int J Biochem Cell Biol. 1996;28:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1074] [Article Influence: 35.8] [Reference Citation Analysis (0)] |