Published online Mar 7, 2005. doi: 10.3748/wjg.v11.i9.1303
Revised: August 14, 2004
Accepted: October 5, 2004
Published online: March 7, 2005
AIM: To identify potential diagnostic target genes in early reperfusion periods following warm liver ischemia before irreversible liver damage occurs.
METHODS: We used two strategies (SSH suppression subtractive hybridization and hybridization of cDNA arrays) to determine early changes in gene expression profiles in a rat model of partial WI/R, comparing postischemic and adjacent nonischemic liver lobes. Differential gene expression was verified (WI/R; 1 h/2 h) and analyzed in more detail after warm ischemia (1 h) in a reperfusion time kinetics (0, 1, 2 and 6 h) and compared to untreated livers by Northern blot hybridizations. Protein expression was examined on Western blots and by immunohistochemistry for four differentially expressed target genes (Hsp70, Hsp27, Gadd45a and IL-1rI).
RESULTS: Thirty-two individual WI/R target genes showing altered RNA levels after confirmation by Northern blot analyzes were identified. Among them, six functionally uncharacteristic expressed sequences and 26 known genes (12 induced in postischemic liver lobes, 14 with higher transcriptional expression in adjacent nonischemic liver lobes). Functional categories of the verified marker genes indicate on the one hand cellular stress and tissue damage but otherwise activation of protective cellular reactions (AP-1 transcription factors, apoptosis related genes, heat shock genes). In order to assign the transcriptional status to the biological relevant protein level we demonstrated that Hsp70, Hsp27, Gadd45a and IL-1rI were clearly up-regulated comparing postischemic and untreated rat livers, suggesting their involvement in the WI/R context.
CONCLUSION: This study unveils a WI/R response gene set that will help to explore molecular pathways involved in the tissue damage after WI/R. In addition, these genes especially Hsp70 and Gadd45a might represent promising new candidates indicating WI/R liver damage.
- Citation: Fallsehr C, Zapletal C, Kremer M, Demir R, von Knebel Doeberitz M, Klar E. Identification of differentially expressed genes after partial rat liver ischemia/reperfusion by suppression subtractive hybridization. World J Gastroenterol 2005; 11(9): 1303-1316
- URL: https://www.wjgnet.com/1007-9327/full/v11/i9/1303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i9.1303
Prolonged warm ischemia followed by reperfusion is a main determinant of hepatic injury in patients undergoing hemorrhagic shock[1], late sepsis[2], trauma[3], liver resection or transplantation[4]. While cold storage that appears to cause injury mainly to sinusoidal endothelial cells[5], WI/R has damaging effects on both, hepatocytes and endothelial cells[6]. Liver damage initiated by transient warm ischemia and aggravated during reperfusion occurs in a biphasic pattern[4,7,8]. In the acute phase (<6 h post ischemia) activated Kupffer cells release reactive oxygen species (ROS) and proinflammatory cytokines. These mediators initiate a complex network of intercellular signalling between endothelial and parenchymal cells[4,9] resulting in platelet and neutrophil recruitment[4,8]. Sinusoidal microcirculation is disturbed due to endothelin-induced into cell contraction and endothelial cell swelling[10]. The subacute reperfusion phase (6-24 h) is characterized by an immense influx of neutrophils, which infiltrate into the liver parenchyma after selectin and immunoglobulin-mediated endothelial adhesion[4,8,11]. Activated neutrophils cause hepatocyte damage by superoxide, ROS[7] and protease[12] release and also via receptor/ligand mediated cell-cell contacts[8]. In addition, ROS can inactivate endogenous protease inhibitors facilitating protease-mediated hepatocyte injury[13]. Consequently irreversible tissue injuries including microcirculatory disturbance[14], sinusoidal cell loss[15] and hepatic cell death[16] may result from WI/R, finally leading to liver dysfunction or failure.
Although much work has been done on certain components, taking part in WI/R liver damage, the exact interactions of mediators and signal transduction in the involved cells remain unclear. One possible way to resolve this complex network is to compare entire gene expression profiles of liver tissues shortly after WI/R before irreversible liver damage occurs and not affecting specimens in the acute reperfusion phase. Apart from general insights into the involved mechanisms, identification of new marker genes with differential screening methods an improve the diagnosis of early WI/R injury and may influence therapeutic strategies. In order to identify such genes, two different methods were used in the present study (SSH and screening of cDNA-arrays) to determine differentially expressed genes in an autologous system of partial rat liver WI/R (1 h/2 h).
Complementary DNA arrays provide a powerful tool to compare gene expression patterns, since many known genes or sequences can be examined in a single experiment[17]. In contrast, the polymerase chain reaction (PCR) based method SSH[18] is a useful technique to compare two entire poly(A)+RNA populations and allows the identification of previously unknown genes. Fragments of digested cDNA, are compared by one round of subtractive hybridization with high subtraction efficiency and normalized representation of abundant or rare, differentially expressed genes. In the subsequent suppression PCR, only differentially expressed sequences are amplified exponentially, while suppression of equally expressed sequences occurs[18].
In the present study, the SSH technique and the hybridization of ‘Atlas Arrays’ (Clontech) were chosen to compare the transcriptional pattern of postischemic liver lobes and adjacent nonischemic liver tissues after 1 h partial ischemia and 2 h reperfusion. After initial screening and validation on Northern blots, 32 differentially expressed genes were identified. Beside genes with known physiological function, six functionally uncharacteristic sequences were identified via SSH, providing a novel source for marker genes indicating WI/R liver damage. Expression profiles of all verified genes were established in detail, comparing healthy rat livers to WI/R-injured tissues in reperfusion time kinetics. Protein expression was investigated for four targets (Hsp70, Hsp27, Gadd45a and IL-1-RI) to correlate the transcriptional status to the protein level.
For the first time, SSH and Atlas array techniques were used in this standard in vivo model of WI/R to identify and verify differentially expressed genes after WI/R that represent potential candidates for the early detection of WI/R liver damage.
Animals and model of WI/R
Rats were housed in two to three per cage with access to water and standard pellet food. The night before surgery, animals were fasted but received water ad libitum. After induction of anaesthesia (Ketamine 10 mg/kg IM and Phenobarbital 18 mg/kg IP), a median laparotomy was performed and the left liver lobe was mobilized. In all animals, warm ischemia of 1 h was induced in the left liver lobe by placing a hemoclip across the pedicle, followed by reperfusion periods of 0, 1, 2 or 6 h. Left and right liver lobes were extracted, snap frozen and stored at -80 °C. Additionally healthy rat livers were explanted directly after laparotomy. During the surgical procedure, body temperature was monitored and maintained at 36.8-37.2 °C using heating pads.
Experiments were approved by the Committee of Animal Care, Regierungspräsidium Karlsruhe, Germany, and performed in accordance to German legislation on protection of animals.
To define the hepatocellular injury, AST and ALT serum levels were measured using routine tests (ADVIA 120, Bayer, Leverkusen).
Formalin-fixed and paraffin-embedded liver sections were stained with hematoxylin and eosin (HE) for conventional morphological evaluation.
RNA was extracted from 40 mg of snap frozen specimens. Twenty micrometers of cryosections were homogenized using QIAshredder columns (Qiagen, Hilden, Germany) and total RNA was isolated with the Rneasy Mini kit (Qiagen). To enrich poly(A)+RNA, we used the Oligotex-Direct mRNA kit (Qiagen). RNA quantification was performed spectrophotometrically and RNA integrity was examined by agarose gel electrophoresis.
SSH was performed as described[18] using reagents supplied with the PCR Select cDNA Subtraction kit (Clontech, Heidelberg, Germany). We used 2.5 μg poly(A)+RNA of the postischemic lobe from one rat liver after partial WI/R (1 h/2 h) and 2.5 μg poly(A)+RNA derived from the corresponding nonischemic liver lobe of the same animal for forward and reverse SSH experiments. PCR products from both SSH experiments were directly cloned into pCR2.1TOPO vector and TOP10F’ cells (Invitrogen, Groningen, Netherlands).
SSH clone inserts were amplified from LB-Kanamycin overnight cultures, using the nested adapter-1 and -2R primers (PCR Select cDNA kit, Table 1), flanking each candidate cDNA after subtraction, as described[18]. In the first screening, PCR products (200 ng/dot) were passed to two identical sets of nylon membranes (GeneScreen, Perkin Elmer Biosystems, Weiterstadt, Germany). In the Southern blot differential approach PCR products were size-fractionated on duplicate agarose gels, including a RT-PCR-amplified β-Actin fragment as positive control on each gel (Table 1), and transferred to nylon membranes (GeneScreen).
| Description | Accession number | Primer sequence (5'-3') | Size1 (bp) | Annealingtemperature (°C) |
| Adapter-1 | TCGAGCGGCCGCCCGGGCAGGT | 68 | ||
| Adapter-2R | AGCGTGGTCGCGGCCGACGT | |||
| β-Actin forward | V01217 | 2161-ATGTTTGAGACCTTCAACAC-2180 | 483 | 56 |
| β-Actin reverse | V01217 | 2743-AACGTCACACTTCATGATGG-2724 | ||
| Fos forward | XM_234422 | 695-TGGTGAAGACCATGTCAGGCG-715 | 404 | 60 |
| Fos reverse | XM_234422 | 1098-ATTGAGAAGAGGCAGGGTGAAGG-1076 | ||
| Jun forward | NM_021835 | 367-GGAAACGACCTTCTACGACGATG-389 | 519 | 60 |
| Jun reverse | NM_021835 | 885-GGGTTGAAGTTGCTGAGGTTGG-864 | ||
| Jund forward | NM_138875 | 465-AGTACGCAGTTCCTCTACCCTAA-487 | 413 | 58 |
| Jund reverse | NM_138875 | 877-ATGTCGATGGGCGACAGTGGA-857 | ||
| Hsp27 forward | M86389 | 157-GTTTCCCGATGAGTGGTCTCAGT-179 | 378 | 63 |
| Hsp27 reverse | M86389 | 534-TCAGGGGACAGGGAAGAGGACAC-512 | ||
| Hsp60 forward | X54793 | 913-CCAGGGTTTGGGGACAACAGG-933 | 380 | 59 |
| Hsp60 reverse | X54793 | 1293-TCTTGTAGCATTGAGAGCATCTG-1271 | ||
| Hsp70 forward | L16764 | 2129-GGCTAGAGACAGACTCTTGATGG-2151 | 276 | 58 |
| Hsp70 reverse | L16764 | 2404-CTCAGTTTGTAGGGATGCAAGG-2383 | ||
| Gadd45a forward | L32591 | 51-GAAGATCGAAAGGATGGACACGG-73 | 307 | 63 |
| Gadd45a reverse | L32591 | 357-GCTCTCAGCGGGGCTCTTGTC-337 | ||
| Ldlr forward | X13722 | 1337-CTGTGGGTTCCATAGGGTTTCTG-1359 | 561 | 60 |
| Ldlr reverse | X13722 | 1897-GTTTGGAATCAACCCAATAGAGG-1875 |
In the first differential screening, complex probes were synthesized from 0.5 µg postischemic and the corresponding nonischemic liver lobe poly (A)+RNA of the SSH starting material using the SMART PCR cDNA Synthesis kit properly (Clontech). A purification step using the High Pure PCR Purification kit (Roche, Mannheim, Germany) was included before random [α-32P]-dCTP (Amersham Pharmacia Biotech, Freiburg, Germany) labeling with 300 ng of the respective cDNAs and HexaLabel DNA Labeling kit (MBI Fermentas, St. Leon-Rot, Germany). For the second screening, we performed complex probes with 400 μg total RNA of four pooled nonischemic and 400 μg total RNA of the four pooled, matched postischemic lobes after partial WI/R (1 h/2 h). Each lobe was represented in equal amounts. RNA-pools were subjected to desoxyribonuclease-I digestion (DNAseI Amp Grade, Invitrogen) before purification of poly(A)+RNA. Linear [α-32P]-dCTP labeling occurred during reverse transcription (Superscript-II, Invitrogen) using 0.5 μg poly(A)+RNA of each RNA-pool as template. Non incorporated nucleotides and primers were separated by NICK Columns (Amersham Pharmacia Biotech).
Equal amounts of [α-32P]-dCTP labeled probes, (1×106 counts/min/mL hybridization solution) were used for hybridization. Membranes were pre-hybridized for 2 h and hybridized for 18 h at 65 °C with heat denatured probes and washed under stringent conditions as described[19]. Filters were exposed with intensifying screens to BioMax MS-1 x-ray films (Kodak).
For Northern blot synthesis 3 μg total RNA was transferred after agarose gel electrophoresis to N+-Hybond membranes (Amersham). Plasmid DNA of SSH clones was prepared from 3 mL LB-Kanamycin overnight cultures using Quantum Prep Plasmid Miniprep kit (Bio-Rad Laboratories, Munich, Germany) for probe generation. Plasmid DNAs (100 ng) were [α-32P]-dCTP labeled by PCR using Taq-polymerase, Mix-C (dATP, dGTP, dTTP, 0.2 mmol/L each), dCTP (0.006 mmol/L), [α-32P]-dCTP (0.8 µCi/µL), sense and antisense primers (Table 1, 0.5 µmol/L each). Adapter-1 and adapter-2R primers were used to label insert DNAs of the chosen SSH clones. Non incorporated [α-32P]-dCTP was removed with High Pure PCR Purification kit columns (Roche). Hybridization procedures as above.
PCR fragments were sequenced with an ABI Prism Big Dye Terminator Cycle Sequencing kit (P/N4303151, Perkin Elmer) and the nested-PCR-primer-1 (Table 1) which binds to adaptor-1 flanking the amplified SSH-fragments, or gene specific primers (Table 1). Sequencing reactions were analyzed on an ABI Prism 310 Genetic Analyzer (Perkin Elmer).
The obtained sequences were compared to DNA databases at the National Center for Biotechnology Information (NCBI) on the BLASTN server (http://www.ncbi.nlm.nih.gov/gorf/bl2.html).
Two ‘Atlas Arrays’ (Atlas Rat cDNA Expression Array #7738-1; Clontech) were hybridized with complex probes generated from 1 µg desoxyribonuclease-I digested poly(A)+RNA preparations as described in the kit manual. Rat RT-PCR-amplified fragments (Table 1) of the resulting candidate genes were cloned in pCR2.1 vector and TOP10’ cells (Invitrogen). Target identity was confirmed after sequencing (see above).
Rat liver cryosections (40 mg) were homogenized in two-fold loading buffer according to the Laemmli system[20] and subjected to SDS polyacrylamide gel electrophoresis. Proteins were transferred on Immobilon-P membranes (Millipore, Eschborn, Germany), blocked in 50 g/L non-fat milk powder and incubated overnight (4 °C) with the following primary antibodies: anti-Hsp70 (1:1000, sc-24, Santa Cruz Biotechnologies, Heidelberg, Germany) anti-Hsp27 (1:2 000, SPA-810, Stressgen, Victoria, Canada), anti-Gadd45a (1:1000, sc-792, Santa Cruz), anti-IL-1RI (1:1000, sc-689, Santa Cruz) or anti-actin-β (#69100, ICN Biomedicals, Eschwege, Germany). Subsequent to washing procedures, filters were incubated for 1 h (25 °C) with horseradish peroxidase-coupled anti-rabbit-IgG or anti-mouse-IgG (W4011; Promega, Mannheim; 315-035-003, Dianova, Hamburg, Germany; 1:10000 each). Signals were visualized by enhanced chemiluminescence (ECL detection kit, Amersham) and exposure to ECL hyperfilms (Amersham).
The LSAB rat kit (DakoCytomation, Hamburg, Germany) was used to stain paraffin embedded liver sections. Immunohistochemistry with cryoconserved tissues was performed with the universal Vectastain Elite ABC kit (Vector, Burlingame, USA). Both procedures were performed according to manufacturer’s instructions. Anti-Hsp70 (1:1000, sc-24, Santa Cruz) and anti-Gadd45a (1:200, sc-792, Santa Cruz) were utilized as primary antibodies.
AST and ALT levels as well as densitometric results of analyzed Northern blot hybridizations are expressed in mean±SE.
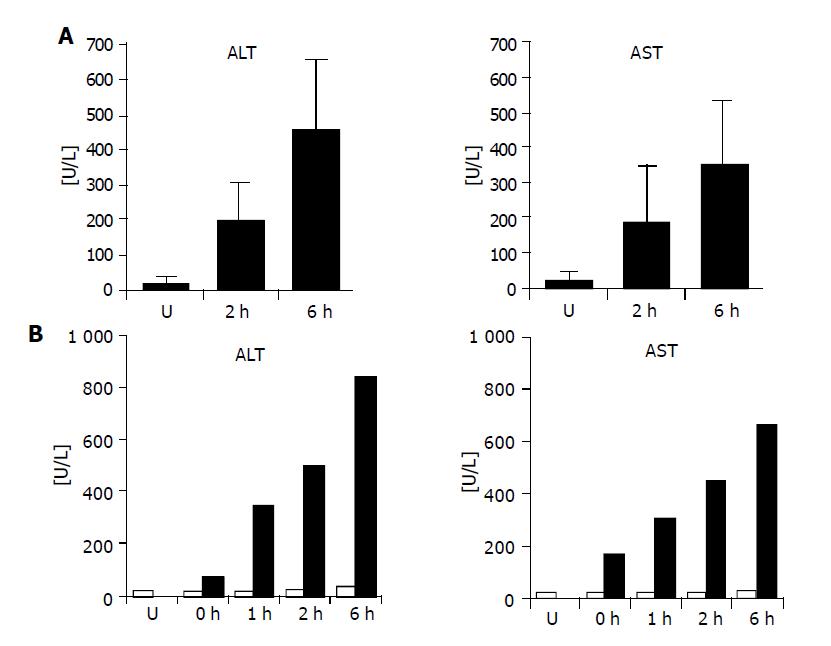
In order to define the reperfusion time point at which liver dysfunction is initiated but morphologically not yet detectable, animals were subjected to 1 h warm ischemia and 2 or 6 h reperfusion (n = 5 per group). Serum aspartate aminotransferase or alanine aminotransferase (AST/ALT) levels were measured and compared to liver enzymes in sera of five untreated animals to assess general liver dysfunction. AST and ALT levels were increased in both experimental groups with higher levels after 6 h reperfusion as compared to healthy animals (Figure 1A). For Northern and Western blot experiments, an additional liver series after 1 h partial ischemia and 0, 1, 2 or 6 h of reperfusion was prepared. To monitor liver damage, serum AST and ALT concentrations were determined before and after WI/R. AST levels were in a normal range before WI/R and in untreated rat livers (AST: 27.8±4.8 u/L; ALT: 23.6±5.7 u/L) but increased in all postischemic specimens, indicating strongest organ damage 6 h post ischemia (AST: 660 u/L; ALT: 840 u/L) (Figure 1B).
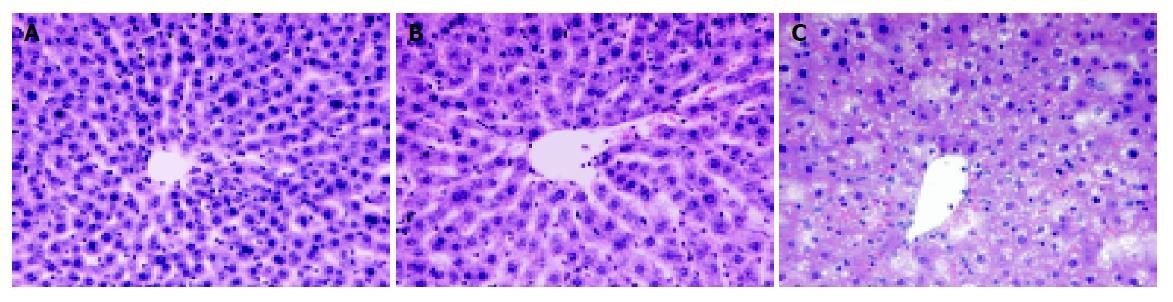
To include WI/R relevant histopathological changes in the postischemic liver tissues HE stained sections were examined for all rat livers. In untreated rats, almost normal liver architecture was observed in histology (Figure 2A). A low number of infiltrated cells were noticed 2 and 6 h post ischemia. Comparing untreated specimens to rat liver lobes after 1 h warm ischemia and 2 h reperfusion, no irreversible liver damage could be detected morphologically (Figure 2B). Vacuolation of hepatocytes and a few single pyknotic or single necrotic cells were restricted to the reperfusion time point 6 h post ischemia representing reversible and irreversible liver destructions, respectively (Figure 2C). However, only mild signs of liver damage were observed, no large necrotic areas could be detected in any analyzed tissue section.
For the identification of differentially expressed genes via SSH and cDNA array hybridizations, 1 h warm ischemia and 2 h reperfusion was chosen as experimental setting.
To compare transcriptional profiles of postischemic (I-lobe) and the adjacent nonischemic liver tissue (C-lobe), two SSH experiments were performed with poly(A)+RNA of one animal subjected to partial liver WI/R (1 h/2 h). A total number of 1565 candidate clones were obtained, among them 809 clones from forward (I-lobe enriched transcripts) and 756 clones from reverse subtraction (C-lobe enriched transcripts). Differential dot blot screening of all 1565 candidate clones was performed using complex probes generated from the same poly(A)+RNA sources, that were used as starting material for SSH experiments.
A total number of 163 clones (10.4%) showed significantly different hybridization signal intensities between the two complex probes. Sequencing analysis revealed that these 163 clones were derived from 89 individual target genes. Of these, 28 genes up-regulated in the examined I-lobe and 61 genes up-regulated in the corresponding C-lobe were subjected to a second screening on reverse Southern blots with complex probes derived from pooled poly(A)+RNA of four I-lobes and pooled poly(A)+RNA of the four matched C-lobes after WI/R (1 h/2 h). β-Actin RT-PCR fragments were chosen as positive controls on each blot. Figure 3A shows representative examples of the differential screening approaches. To monitor transcriptional differences, hybridization volumes were normalized to the β-Actin signal of the individual blots, before we originated the ratios of pool-C vs pool-I hybridized signals. Figure 3C summarizes signal differences after densitometric measurements of the 89 analyzed genes. Gene selection for further analysis could be subdivided into seven genes with higher relative cDNA amounts in the pooled I-lobes (pool-C/pool-I ≤0.5), 20 clones showing higher transcriptional level in pool-C (pool-C/pool-I ≥2) and five noninformative candidates.
Two Clontech ‘Atlas Array’, consisting of spotted cDNAs corresponding to 588 functionally known genes, were hybridized with pooled cDNA of matched postischemic and nonischemic rat liver lobes. We started with 1 µg poly(A)+RNA pools of the four rat livers used for the secondary SSH screening after WI/R (1 h/2 h). A comparison of signal intensities after normalization against β/γ-Actin and the gene coding for the ribosomal protein S29 revealed 17 genes up-regulated in the I-lobe pool and 13 genes up-regulated in the C-lobe pool. To compensate the low number of I-lobe enriched targets, resulting from the SSH screening, ten up-regulated and three down-regulated candidate genes (in the I-lobes) were selected for further investigation. Figure 3D shows the hybridization result of the two ‘Atlas Array’ after 4 h exposition.
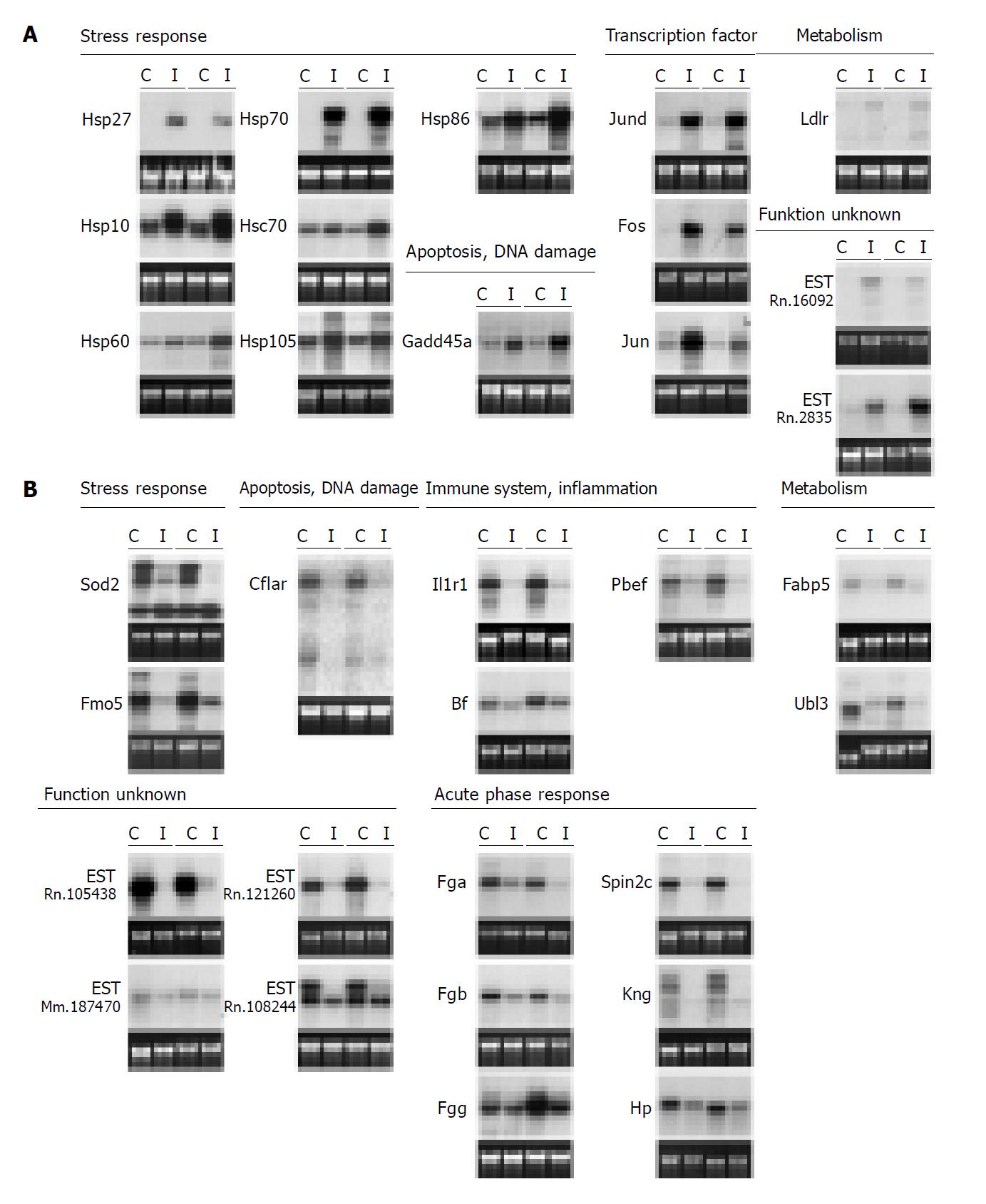
After the long run of screening procedures, we further analyzed 42 candidate genes obtained from SSH and ‘Atlas Array’ experiments (including three genes identified with both methods) by Northern blot hybridizations. Gene expression was analyzed with RNA of up to four paired C- and I-lobes, extracted after partial WI/R (1 h/2 h).
In total, 32 genes (76.2%) showed a reproducible differential expression pattern (for ≥3 matched pairs). Fourteen genes were up-regulated in the I-lobes while 18 genes showed higher expression in the corresponding C-lobes. Mean fold increase of the paired lobes ranged from 1.4 to 74.3. Figure 4 shows the hybridization results for all 32 differentially expressed genes for two postischemic and the two matched nonischemic liver lobes. Table 2 and table 3gives an overview of the differentially expressed genes including official gene name abbreviations and densitometric results of the analyzed Northern blots. The differentially expressed genes can be divided into seven groups, according to their physiological functions of their encoded proteins (Table 2). They include genes that are involved in cellular stress response, DNA damage and apoptosis, genes coding for transcription factors of the AP-1 family, genes coding for immune response proteins as well as acute phase proteins and proteins involved in cellular metabolism. The last category comprises expressed but functionally not characterized sequences.
| Description | Up-regulated genes (C-lobes vs I-lobes; n = 14) | ||||||
| UniGene ID | Accession number | % Identity1 | Method | Mean fold increase2 | SE3 | WI/R citation4 | |
| Stress response | |||||||
| Heat shock 10 ku protein 1 (Hspe1,Hsp10) | Rn.106093 | NM_012966 | id | SSH | 2.8 (n = 4) | 1.4 | |
| Heat shock protein 27 ku (Hsp27) | Rn.3841 | M86389 | id | A | 13.7 (n = 4) | 1.6 | [21, 27] |
| Heat shock protein 60 ku (liver) (Hsp60) | Rn.102058 | X54793 | id | A | 1.4 (n = 4) | 1.4 | |
| Heat shock 70 ku protein 1A (Hspa1a,Hsp70) | Rn.1950 | L16764 | id | SSH/A | 64.0 (n = 4) | 1.6 | [22, 36-39] |
| Heat shock 70 ku protein 8 (Hspa8, Hsc70) | Rn.120392 | M11942 | id | SSH | 1.5 (n = 4) | 1.5 | [37, 40] |
| Heat shock protein 1, alpha (Hspca,Hsp86) | Rn.3277 | AJ297736 | id | SSH | 2.5 (n = 4) | 1.1 | [39] |
| Similar to heat shock protein 105 ku alpha (LOC288444, Hsp105) | Rn.37805 | XM_213699 | id | SSH | 1.9 (n = 4) | 1.1 | |
| Cell cycle arrest, DNA damage, apoptosis | |||||||
| Growth and DNA-damage-inducible transcript-1 (Gadd45a) | Rn.10250 | L32591 | id | A | 3.7 (n = 4) | 1.2 | |
| Transcription factor | |||||||
| Jun D proto-oncogene (Jund) | Rn.46225 | NM_138875 | id | A | 4.6 (n = 4) | 1.3 | [9, 29] |
| c-fos mRNA (Fos) | Rn.103750 | XM_234422 | id | A | 16.3 (n = 4) | 1.9 | [9, 28, 29, 36, 37, 41] |
| Avian sarcoma virus 17 oncogene homolog (Jun) | Rn.93714 | NM_021835 | id | A | 2.5 (n = 3) | 1.5 | [9, 28, 29, 36, 41] |
| Cellular metabolism | |||||||
| Low density lipoprotein receptor (Ldlr) | Rn.10483 | X13722 | id | A | 9.1 (n = 4) | 1.4 | |
| Gene product function unknown | |||||||
| ESTs | Rn.16092 | BM391881 | id | SSH | 5.2 (n = 4) | 1.2 | |
| ESTs | Rn.2835 | BG664888 | id | SSH | 3.6 (n = 4) | 1.4 | |
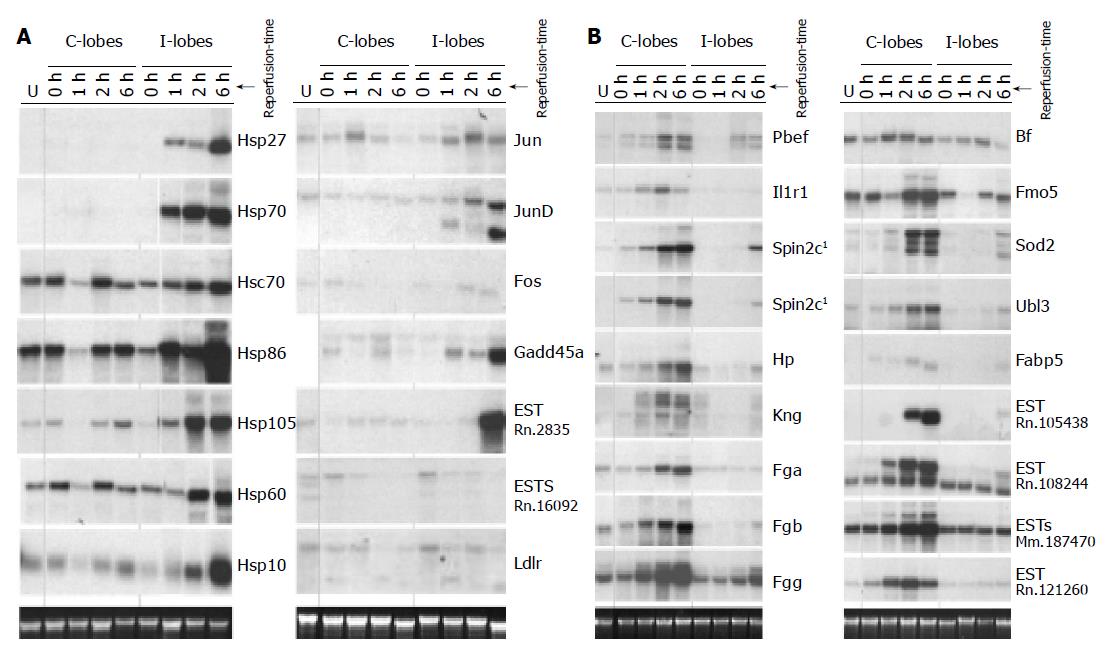
To establish more comprehensive gene expression profiles of the 32 identified targets after partial ischemia we analyzed RNA of untreated rat livers as well as RNA of the additionally prepared liver series after 1 h partial ischemia and 0, 1, 2 or 6 h of reperfusion on Northern blots.
Figure 5A shows the resulting Northern blot data for the 14 I-lobe up-regulated genes. Ten genes were identified that were clearly up-regulated in the matched postischemic lobes as compared to the untreated livers and the C-lobes of the experimental animals: Hsp27, Hsp70, Hsp86, Hsp105, Hsp60, Hsp10, Jund, Fos, Gadd45a and EST-Rn.2835. All analyzed heat shock genes as well as Jund, Gadd45a and EST-Rn.2835 showed highest transcriptional levels after 6 h reperfusion equal to the highest serum AST/ALT levels in the analyzed reperfusion time kinetics (compare Figure 5A to Figure 1B). Two genes, Ldlr and EST-Rn.160092, showed higher expression levels in the untreated livers as well as in the I-lobes after 2 h reperfusion, whereas in the matched C-lobes RNA levels were lower. The reperfusion time kinetics indicates that these two genes are down-regulated with ongoing reperfusion time and liver damage; this effect was seen earlier in the C-lobes than in the I-lobes (Figure 5A).
Figure 5B demonstrates the transcription levels of 17 genes up-regulated in the C-lobes. Twelve genes have comparably low RNA levels in the analyzed I-lobes and the untreated rat livers but are clearly induced in the C-lobes, comprising of genes coding for plasma proteins including acute phase genes, Pbef, IL-1RI, Sod2, Ubl3, Fabp5 as well as three ESTs. Except for the genes coding for IL-1RI, Bf, Sod2 and the EST-Rn.121260, all genes with induced expression in the C-lobes showed highest transcriptional levels 6 h post ischemia. Figure 5B also presents some genes with delayed increased gene expression in the I-lobes compared to the corresponding C-lobes after 6 h reperfusion and untreated rat livers (Pbef, Spin2c, Fgg, Sod2, Fabp5, EST-Rn.121260 and EST-108244).
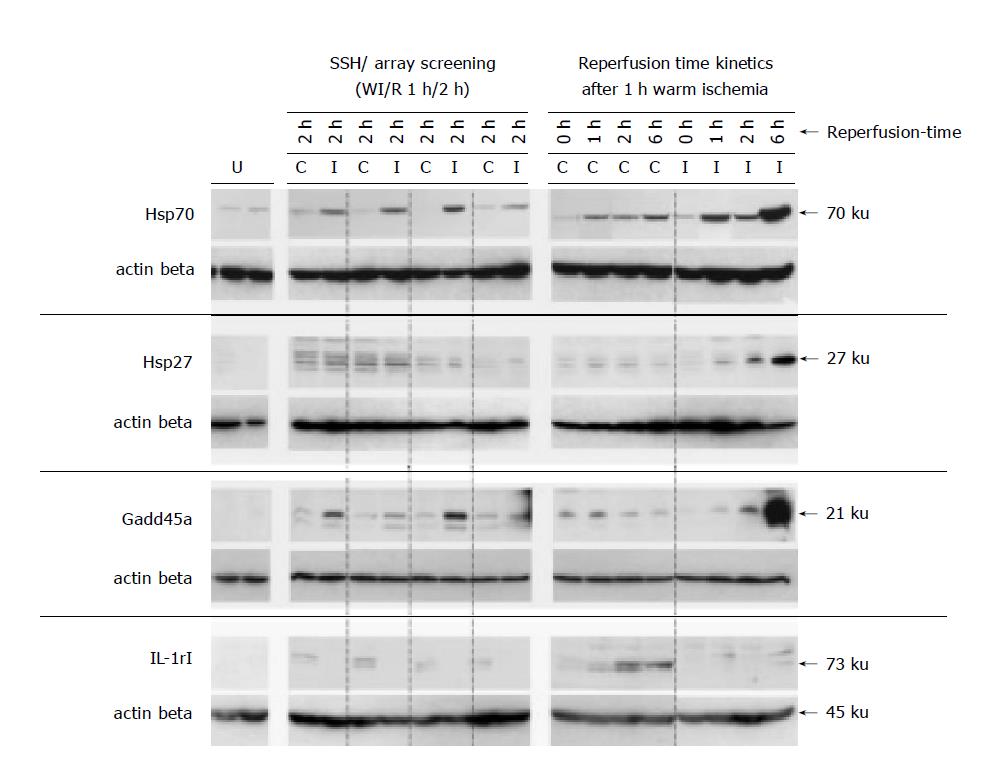
We analyzed the protein levels of four verified target genes (Hsp70, Hsp27, Gadd45a and IL-1RI) on Western blots after 1 h partial ischemia and 2 h reperfusion (n = 3), in the specimens of the representative reperfusion time kinetics (0, 1, 2 and 6 h reperfusion; n = 1) and untreated rat livers (n = 2) as shown in Figure 6.
Although increased transcript levels of the Hsp27 gene were observed after 1 h reperfusion (Figure 5A), protein levels were elevated slightly in the I-lobes after 2 h and more pronounced after 6 h of reperfusion. Furthermore, Figure 6 shows increased IL-1RI amounts in the analyzed nonischemic lobes (1, 2 and 6 h reperfusion) compared to protein levels in the corresponding postischemic lobes and untreated livers.
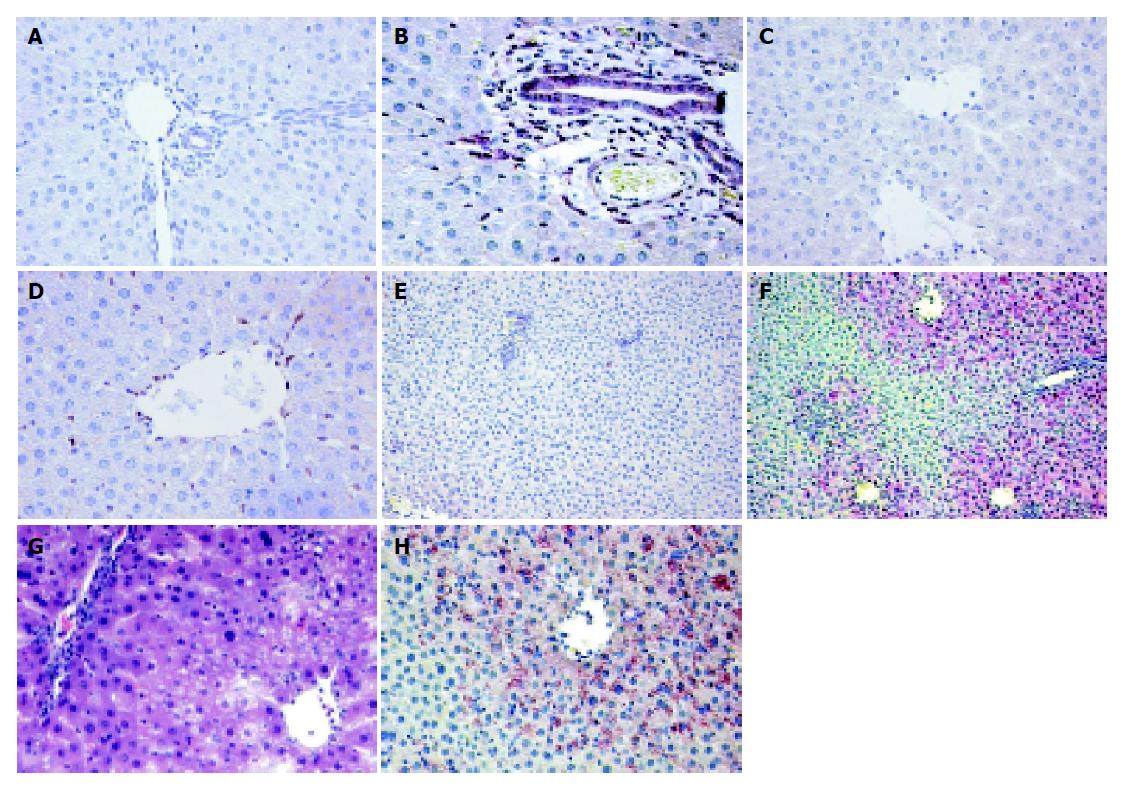
Increased Gadd45a protein could be detected as early as after 2 h of reperfusion but was more elevated after 6 h of reperfusion in the postischemic liver lobes as compared to untreated rat livers and respective C-lobes (Figure 6). Immunohistochemical Gadd45a staining of fresh frozen liver tissues demonstrated induced Gadd45a expression after 2 and 6 h of reperfusion, with cytoplasmic and nuclear staining in parenchymal cells (Figure 7). In untreated livers, in all analyzed C-lobes as well as in postischemic I-lobes up to 1 h of reperfusion, Gadd45a expression was absent or at basal levels (data not shown).
Hsp70 protein was clearly induced in the I-lobes after 1, 2 and 6 h of reperfusion (compared to the protein level of the adjacent C-lobes and untreated rat livers), as demonstrated by the Western blot experiments shown in Figure 6. Slightly up-regulated Hsp70 protein levels could also be noticed in the C-lobes with advanced reperfusion time compared to untreated, healthy rat livers. Looking at the immunohistochemical data, Hsp70 induction was observed 1 and 2 h after clamp removal in the analyzed I-lobes only in bile ducts, connective tissue and endothelial cells in portal fields (Figure 8). Additionally, positive staining was noticed in sinusoidal endothelial cells (SEC) and cells in the liver sinusoids. In the analyzed early reperfusion periods (WI/R 1 h/1 h or 1 h/2 h), no Hsp70 expression could be detected in hepatocytes, in contrast to the postischemic liver lobe after 6 h reperfusion. Here intense Hsp70 staining was mainly restricted to parenchymal cells in perivenular liver arreals (Figure 8). In comparison to histopathological changes after WI/R, parenchymal vacuolation correlated with the intense perivenular Hsp70 staining pattern in the I-lobe at this time point (Figure 8).
To date, no reliable parameters exist to allow early prediction of liver damage after hepatic resection or transplantation. Conversely, it would be of great clinical importance to have information about potential function of the liver remnant or graft. In this study we established gene expression profiles of 32 genes with differential expression in the acute phase after partial rat liver WI/R, in order to elucidate the genes and molecular pathways involved in the complex setting of WI/R mediated liver injury. For the first time, the two methods SSH and hybridization of ‘Atlas Array’ were used to obtain a comprehensive profile of differentially expressed genes in this standard WI/R animal model. To analyze, if genes coding for functionally related proteins show similar expression patterns, steady state RNA levels were compared in ischemic/reperfused vs adjacent control liver lobes and healthy livers by Northern blot analysis. These gene patterns should reveal new makers for early WI/R liver injury.
In case of the SSH scenery, candidate genes were first screened with the raw material used for the SSH experiments and subsequently with pooled poly(A)+RNA of four rat livers after 1 h/2 h WI/R, to reduce the number of candidate genes from 89 to 32. This second screening allows the comparison of SSH and ‘Atlas Array’ approaches, because the same pooled RNA source was used for both screening methods. Both procedures yielded a comparable set of differentially expressed genes. The slightly higher rate of differentially expressed candidates of 5.1% recovered from the ‘Atlas Array’ compared to 1.7% after SSH may result from the restriction of the spotted cDNAs on the arrays. The cDNA arrays used here include many gene families involved in signal transduction, cellular stress and inflammation pathways, known to play a role during WI/R, in contrast to the SSH-clones representing a potential of differentially expressed genes from a more unfiltered background. This fact could also explain why only three identical genes were identified with both methods (Hsp70, IL-1rI and Fgb). While the array technology allowed a faster identification of candidate genes, SSH yielded six functionally noncharacterized but via Northern hybridizations confirmed sequences, that were not included in any arrays, allowing for the detection of new marker genes in the WI/R context. After Northern blot analysis, differential expression could be confirmed for 76.9% (11/13) of ‘Atlas Array’ candidate genes and for 78.1% (25/32) of SSH candidate genes after WI/R (1 h/2 h), that included 26 genes or sequences differentially expressed in matched liver lobes of more than three animals with fold changes of relative transcript amounts ≥2.0 (Table 2). In addition to genes that were already described in experimental or clinical settings to be involved in WI/R liver injuries (Table 2), we verified transcriptional differential expression of 11 functionally known genes not described in this in vivo liver WI/R model before, but generally belonging to gene families that are known to be effected after WI/R. These genes were: Hsp10, Hsp60, Hsp105 (coding for heat shock proteins), Gadd45a (apoptosis), Ldlr (metabolism) that were all induced in the clamped liver lobes and Fmo5 (stress responsive gene), Cflar (apoptosis inhibitor), Pbef and Spin2c (immune system, acute phase gene), Ubl3 and Fabp5 (cellular metabolism) with higher transcriptional levels in the analyzed C-lobes. Additionally, we showed for the first time transcriptional differences between postischemic and nonischemic lobes of the genes Hsp27, Jund, Sod2 and IL-1rI known to be involved in WI/R liver injury whose transcriptional status has so far remained unclear from literature data.
After the more detailed gene expression profiling including reperfusion time kinetics and livers of untreated animals (Figure 5), a more in-depth picture of gene expression was seen. First, up-regulated gene expression in one of the experimental groups (C- or I-lobes) vs healthy rat livers occured rather in early reperfusion phases than in the ischemic period (except Spin2c and EST-Rn.121260) that may be due to high energy nucleotide depletion in the ischemic period[4,9]. This finding also explains the delayed up-regulated gene expression in the I-lobes compared to the corresponding C-lobes after 6 h reperfusion and untreated rat livers for seven analyzed sequences. Secondly, genes coding for functionally related proteins show similar expression patterns in the analyzed tissue specimens that is now discussed in detail below.
Figure 5A summarizes the genes of main interest that are up-regulated in the postischemic lobes (I-lobes) compared to the matched C-lobes as well as to the normal livers. Eleven genes could be assigned to genes coding for heat shock proteins, one to apoptosis inductor and one belongs to the AP-1 transcription factor group.
Also known as molecular chaperones, the heat shock gene product family members are responsible to maintain correct protein folding; they are in part induced by denatured proteins produced during heat shock, ischemia or chemical noxes[21]. Interestingly, all important cytoplasmic, nuclear and mitochondrial chaperone systems were involved in our model of WI/R. Hsp70 the major inducible cellular chaperone and Hsp27, a member of the heterogeneous family of small heat shock proteins[21], showed the strongest up-regulation in our model. Hsp70 is accepted to be a sensitive marker indicating WI/R induced cellular stress during the early and subacute reperfusion phase. Hsp70 maintains correct protein folding[22,23] and has protective effects after chemical and ischemic preconditioning followed by liver WI/R[21,24]. It has been shown recently that Hsp70 is a potent inhibitor of apoptotic cell death, additionally explaining the protective effects after preconditioning inducing heat shock protein expression[23,25]. In our experiments, Hsp70 and Hsp27 gene transcripts were nearly absent in untreated rats and C-lobes after WI/R but were up-regulated in the I-lobes. Nearly all published Hsp70 protein analyzes after WI/R do not include Hsp70 localization, but focus on ELISA or Western blot data. Our immunohistochemical results with Hsp70 restriction to ductal cells and SEC 1-2 h postischemia as well as intense parenchymal Hsp70 expression 6 h after reoxygenation of the clamped liver lobe, reflect the time course of this WI/R model[4]. This parenchymal Hsp70 protein staining 6 h after warm liver ischemia in correlation to hepatocyte vacuolation shows that Hsp70 is a sensitive marker for WI/R liver damage. Interestingly, the perivenular Hsp70 distribution could also be demonstrated in rat livers 48 h after heat shock exposure[26]. In the case of Hsp27, for the first time we demonstrated a significant protein up-regulation after partial liver WI/R. In experimental settings after kidney I/R it could be shown that Hsp27 is phosphorylated and activated after I/R; activated-p38 was involved in phosphorylation of Hsp27[27].
In the case of AP-1 transcription factors, which are involved in cellular regeneration or apoptosis[9,28], the immediate early genes Fos, Jun and Jund were up-regulated after WI/R. Fos and Jun gene expressions are known to be induced in the acute reperfusion phase after WI/R[28]. Our transcriptional data, concerning these two genes, are in agreement with the published ones. Furthermore, it could be shown that DNA binding activity of AP-1 dramatically increased in the acute reperfusion phase 1-3 h post-ischemia[29], predominantly of Jun and Jund hetero- and homodimers. AP-1 activity with Jund was mainly found to be up-regulated due to posttranslational modifications regulating rather anti-proliferative responses after WI/R[9,29]. This feature is underscored by the observation that levels of proliferating cell nuclear antigen (a proliferation marker) decrease in concordance with elevated Jund[28]. Now we close the circle and demonstrate transcriptional induction of Jund poly(A)+RNA in the postischemic tissues after partial WI/R. Acting as a transcriptional complex, the up-regulation of these immediate early response genes can cooperatively induce the expression of inflammatory cytokines. This leads to neutrophil-mediated inflammation[9], thereby linking acute molecular events with early cellular damaging effects to the subacute inflammatory responses.
Gadd45a gene is regulated in correlation with the induction of apoptosis or as a consequence of stress response[30]. In our experiments, the Gadd45a gene was induced in the postischemic tissues ≥1 h on RNA level and ≥2 h on protein level. Gadd45a may play a role in p53 dependent and independent apoptosis[30] via activation of JNK and/or p38-MAPK signalling pathways[31]. Both are known to be part of signal transduction cascades in the acute reperfusion phase after liver WI/R[9]. Elevated apoptotic rates are recognized after WI/R although necrosis appears to be the principal mechanism of cell death after WI/R[16].
Looking at the different pathophysiological sequences after liver ischemia[4] as described in the introduction, reperfusion injury is initiated in the early or immediate reperfusion period (1-6 h) with activation of Kupffer cells and induction of microcirculatory disturbance. These changes lead to immense neutrophil inflammatory response, parenchymal apoptosis and more pronounced necrosis in the later postischemic periods (>6 h)[16]. With the identification of genes already up-regulated 1-2 h post-liver clamping in the manipulated liver tissues (I-lobes), compared to the analyzed normal liver tissue, these marker genes indicate not only cellular stress but also tissue injury in the early reperfusion phase (1-2 h) with progressive aggravation. Considering our presented protein data, cellular stress is indicated by the chaperones Hsp27, Hsp70 and the apoptosis or growth arrest inducing protein Gadd45a, with clearly but slightly elevated protein levels in the early reperfusion period in the postischemic liver lobes and immense up-regulated protein synthesis at the later stage (6 h reperfusion).
In contrast to the discussed I-lobe induced genes, Figure 5B combines 18 sequences primarily identified as verified down-regulated after partial WI/R, comparing I-lobes and the corresponding C-lobes of our standard model (WI/R; 1 h/2 h), showing an interesting feature after expression profiling in contrast to the untreated specimens. These gene transcripts were enriched in the C-lobes that were under permanent blood flow throughout the whole of surgical procedure, with some genes indicating a delayed up-regulation in the post ischemic tissues (I-lobes) after 6 h reperfusion. This indicates that various mediators or direct effects of ROS, produced in the clamped liver lobe, systemically influence the adjacent perfused liver lobes during the ischemic period or subsequent to reperfusion. It is well known that TNFα and IL-1 and other mediators have effects not only on the local hepatic environment, but also on remote organs after WI/R[8,32,33], representing the main mechanism of multiorgan dysfunction after liver transplantation and resection[34]. Additionally, it could be shown that blocking IL-1R with its physiological antagonist (IL-1Ra) has beneficial effects after WI/R[35-50]. Our findings demonstrating the up-regulation of acute-phase response/inflammation genes in the adjacent C-lobes in contrast to the corresponding I-lobes after partial WI/R and to the untreated animals support this hypothesis, most notably, increased IL-1RI RNA and protein synthesis in the analyzed C-lobes (≥1 h post ischemia). IL-1RI interacts with IL-1 and TNFα leading to inflammatory responses via NFκB activation[4,8,11]. Another source for mediators leading to altered gene expression in the C-lobes could be the splanchnic region due to portal hypertension as a result of incomplete decompression via the unclamped liver lobes in the case of partial WI/R, i.e., endotoxins, that are absorbed by Kupffer cells leading to the activation of these macrophages[32].
In conclusion, we present a new set of genes responsive to WI/R in early reperfusion periods before irreversible liver damage occurs. The fact that these genes (six noncharacterized sequences and 11 genes described for the first time in the liver WI/R context) include members of known involved pathways (heat shock activation, AP-1 involvement, apoptosis, and inflammatory response) confirms that the methods used in this study provide good tools to identify new WI/R indicating genes. It becomes clear that in these early reperfusion periods (1-2 h) destructive mechanisms mediated by up-regulation of proapoptotic genes are counteracted by the pronounced expression of protective acting genes (i.e., heat shock genes). The question is which side will prevail.
As we analyzed IL-1RI, Gadd45a and Hsp27 exemplarily and for the first time in the liver WI/R context on the protein level, future studies should expand protein expression data of the identified marker genes. Differentially expressed ESTs need to be further characterized in experimental and clinical settings especially during liver transplantation and resection. The involvement of different functional compartments should stimulate the definition of a characteristic set of early WI/R markers to distinguish between reversible and irreversible injuries after liver surgery rather than to focus on single markers. Clearly, heat shock proteins as well as AP-1 and apoptosis-related genes and proteins should be part of this diagnostic spectrum.
We thank Dr. Jürgen Kopitz and Dr. Nicolas Wentzensen for helpful discussions and advice.
Assistant Editor Guo SY Edited by Gabbe M
| 1. | Clemens MG, Bauer M, Gingalewski C, Miescher E, Zhang J. Hepatic intercellular communication in shock and inflammation. Shock. 1994;2:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | McCuskey RS, Urbaschek R, Urbaschek B. The microcirculation during endotoxemia. Cardiovasc Res. 1996;32:752-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Pachter HL, Spencer FC, Hofstetter SR, Liang HG, Coppa GF. Significant trends in the treatment of hepatic trauma. Experience with 411 injuries. Ann Surg. 1992;215:492-500; discussion 500-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. [PubMed] |
| 5. | Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. Hepatology. 1989;10:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Vizzotto L, Vertemati M, Degna CT, Aseni P. Liver transplantation in man: morphometric analysis of the parenchymal alterations following cold ischaemia and warm ischaemia/reperfusion. J Anat. 2001;198:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 241] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 356] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med (Berl). 1999;77:577-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Pannen BH. New insights into the regulation of hepatic blood flow after ischemia and reperfusion. Anesth Analg. 2002;94:1448-1457. [PubMed] |
| 11. | Martinez-Mier G, Toledo-Pereyra LH, Ward PA. Adhesion molecules in liver ischemia and reperfusion. J Surg Res. 2000;94:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Jaeschke H, Smith CW, Clemens MG, Ganey PE, Roth RA. Mechanisms of inflammatory liver injury: adhesion molecules and cytotoxicity of neutrophils. Toxicol Appl Pharmacol. 1996;139:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647-653. [PubMed] |
| 14. | Menger MD, Richter S, Yamauchi J, Vollmar B. Role of microcirculation in hepatic ischemia/reperfusion injury. Hepatogastroenterology. 1999;46 Suppl 2:1452-1457. [PubMed] |
| 15. | Henderson JM. Liver transplantation and rejection: an overview. Hepatogastroenterology. 1999;46 Suppl 2:1482-1484. [PubMed] |
| 16. | Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 298] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 17. | Lockhart DJ, Winzeler EA. Genomics, gene expression and DNA arrays. Nature. 2000;405:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1426] [Cited by in RCA: 1151] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 18. | Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025-6030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2262] [Cited by in RCA: 2003] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 19. | Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5113] [Cited by in RCA: 6060] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 20. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185380] [Cited by in RCA: 188835] [Article Influence: 3433.4] [Reference Citation Analysis (0)] |
| 21. | Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Flohé S, Speidel N, Flach R, Lange R, Erhard J, Schade FU. Expression of HSP 70 as a potential prognostic marker for acute rejection in human liver transplantation. Transpl Int. 1998;11:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Sakai T, Takaya S, Fukuda A, Harada O, Kobayashi M. Evaluation of warm ischemia-reperfusion injury using heat shock protein in the rat liver. Transpl Int. 2003;16:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Doi Y, Hamazaki K, Yabuki M, Tanaka N, Utsumi K. Effect of HSP70 induced by warm ischemia to the liver on liver function after partial hepatectomy. Hepatogastroenterology. 2001;48:533-540. [PubMed] |
| 25. | Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665-25671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 340] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Matsumoto K, Honda K, Kobayashi N. Protective effect of heat preconditioning of rat liver graft resulting in improved transplant survival. Transplantation. 2001;71:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Kumar Y, Tatu U. Stress protein flux during recovery from simulated ischemia: induced heat shock protein 70 confers cytoprotection by suppressing JNK activation and inhibiting apoptotic cell death. Proteomics. 2003;3:513-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Schlossberg H, Zhang Y, Dudus L, Engelhardt JF. Expression of c-fos and c-jun during hepatocellular remodeling following ischemia/reperfusion in mouse liver. Hepatology. 1996;23:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IkappaB degradation. Hepatology. 1998;28:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Sheikh MS, Hollander MC, Fornance AJ. Role of Gadd45 in apoptosis. Biochem Pharmacol. 2000;59:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 606] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 32. | Wanner GA, Ertel W, Müller P, Höfer Y, Leiderer R, Menger MD, Messmer K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 203] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Yoshidome H, Lentsch AB, Cheadle WG, Miller FN, Edwards MJ. Enhanced pulmonary expression of CXC chemokines during hepatic ischemia/reperfusion-induced lung injury in mice. J Surg Res. 1999;81:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Colletti LM, Green M. Lung and liver injury following hepatic ischemia/reperfusion in the rat is increased by exogenous lipopolysaccharide which also increases hepatic TNF production in vivo and in vitro. Shock. 2001;16:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Shito M, Wakabayashi G, Ueda M, Shimazu M, Shirasugi N, Endo M, Mukai M, Kitajima M. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation. 1997;63:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Tacchini L, Schiaffonati L, Pappalardo C, Gatti S, Bernelli-Zazzera A. Expression of HSP 70, immediate-early response and heme oxygenase genes in ischemic-reperfused rat liver. Lab Invest. 1993;68:465-471. [PubMed] |
| 37. | Schiaffonati L, Rappocciolo E, Tacchini L, Cairo G, Bernelli-Zazzera A. Reprogramming of gene expression in postischemic rat liver: induction of proto-oncogenes and hsp 70 gene family. J Cell Physiol. 1990;143:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Gingalewski C, Theodorakis NG, Yang J, Beck SC, De Maio A. Distinct expression of heat shock and acute phase genes during regional hepatic ischemia-reperfusion. Am J Physiol. 1996;271:R634-R640. [PubMed] |
| 39. | Gasbarrini A, Esposti SD, Di Campli C, De Notariis S, Loffredo S, Abraham A, Simoncini M, Pola R, Colantoni A, Trevisani F. Effect of ischemia--reperfusion on heat shock protein 70 and 90 gene expression in rat liver: relation to nutritional status. Dig Dis Sci. 1998;43:2601-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Bernelli-Zazzera A. Stress proteins and the liver. Ital J Gastroenterol. 1993;25:49-51. [PubMed] |
| 41. | Tacchini L, Radice L, Bernelli-Zazzera A. Differential activation of some transcription factors during rat liver ischemia, reperfusion, and heat shock. J Cell Physiol. 1999;180:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Gupta M, Dobashi K, Greene EL, Orak JK, Singh I. Studies on hepatic injury and antioxidant enzyme activities in rat subcellular organelles following in vivo ischemia and reperfusion. Mol Cell Biochem. 1997;176:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology. 2001;33:902-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Engelhardt JF. Redox-mediated gene therapies for environmental injury: approaches and concepts. Antioxid Redox Signal. 1999;1:5-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Hauptmann G, Tongio MM, Klein J, Mayer S, Cinqualbre J, Jeanblanc B, Kieny R. Change in serum properdin factor B phenotype following human orthoptic liver transplantation. Immunobiology. 1980;158:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Maury CP, Teppo AM, Höckerstedt K. Acute phase proteins and liver allograft rejection. Liver. 1988;8:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Schuster R, Otto G. Behavior of serum proteins after experimental liver transplantation in swine. Z Exp Chir. 1980;13:339-344. [PubMed] |
| 48. | Müller HE, Lie TS. The metabolism of 28 serum proteins in a human liver transplantation (author's transl). Z Immunitatsforsch Exp Klin Immunol. 1973;145:250-274. [PubMed] |
| 49. | Otto G, Grossmann H. Serum protein changes in liver transplantation in the dog. Z Exp Chir. 1976;9:136-140. [PubMed] |
| 50. | Scholz T, Backman L, Mathisen O, Buø L, Karlsrud T, Johansen HT, Bergan A, Klintmalm GB, Aasen AO. Activation of the plasma contact system and hemodynamic changes after graft revascularization in liver transplantation. Transplantation. 1995;60:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |