INTRODUCTION
RNA interference (RNAi) is a widely conserved phenomenon of specific gene silencing among nearly all eukaryotes[1-4]. Studies of model systems such as Caenorhabditis elegans and Drosophila have revealed most of its mechanisms. The long double stranded RNA (dsRNA) is processed by RNase III enzyme Dicer into small interfering RNAs (siRNAs) about 21-23 bp in length[5]. The siRNA is then incorporated into a multi-component RNA-induced silencing complex (RISC), which specifically degrades the homologous mRNA[6]. Because of its high efficiency and specificity, RNAi is now widely used to suppress gene expression. Great number of human disease-related genes have been targeted by RNAi[7-10].
Hepatitis B virus (HBV) infection is a worldwide health problem. Chronic HBV infection will lead to severe liver diseases such as liver fibrosis, liver cirrhosis and hepatocellular carcinoma[11]. Current treatments for HBV infection are low efficient and faced with problems including the emergence of drug resistant mutants[12,13]. Therefore novel therapeutic strategies are urgently needed. Shlomai et al first demonstrated that siRNAs produced intracellularly under the control of an RNA polymerase III (Pol III) promoter can inhibit HBV replication in cell culture[14]. Hamasaki et al reported of the inhibition of HBV replication by synthesized siRNAs in vitro[15]. By cotransfection of HBV replication competent plasmids with plasmids expressing short hairpin RNAs (shRNAs) via hydrodynamic injection, HBV replication was effectively suppressed in the mouse liver[16]. Much more successful preclinical studies are reported[17-22]. However, some obstacles still need to be overcome before RNAi can be brought into clinical use, especially the economical problem of chemically synthesizing siRNAs. Two alternative methods have been discovered so far. One is transcribing long dsRNAs in vitro and digesting with Escherichia coli RNase III or dicer[23-25], the other is digesting in vitro transcribed single stranded RNAs with deoxyribozyme followed by annealing[26]. Here we describe a novel and more cost-effective method for siRNA preparation and its application in inhibition of the replication of hepatitis B virus in HepG2 cells.
MATERIALS AND METHODS
Reagents and materials
The alkaline phosphatase expression vector pSEAP2-control and the enhanced green fluorescent protein expression vector pEGFP-N2 were purchased from CLONTECH (USA). Cloning vector pUCmT was purchased from Shanghai SANGON (China). pET-30a and Ni-NTA His•Bind Resin were purchased from Novagen (USA). QIAGEN ® Plasmid Midi Kit was purchased from QIAGEN (Germany). Whatman® fibrous cellulose powder CF 11 was purchased from Whatman (USA). Diagnostic kit for hepatitis B Surface antigen and e antigen (ELISA) were purchased from Shanghai SIIC KEHUA (China). RNase A and RNase III were purchased from Ambion (USA). Lipofectamine 2000 was obtained from Invitrogen Lifetechnology (USA).
Construction of hepatitis B virus replication competent plasmid
Genomic DNA of HBV adr type was digested with BamH I and inserted into pUCmT vector between Bgl II and BamH I sites, and another copy of the linearized genome was inserted into the resulting plasmid at BamH I site. The obtained plasmid pUC-HBV2 containing duplicate HBV-DNA in head-to-tail orientation was verified by amplification of the HBX coding sequence using forward oligonucleotide 5’-ggaattcatggctgctaggctgtg-3’ and reverse oligonucleotide 5’-ggggtaccggcagaggtgaaaaagttg-3’.
Construction of dsRNA expressing vectors
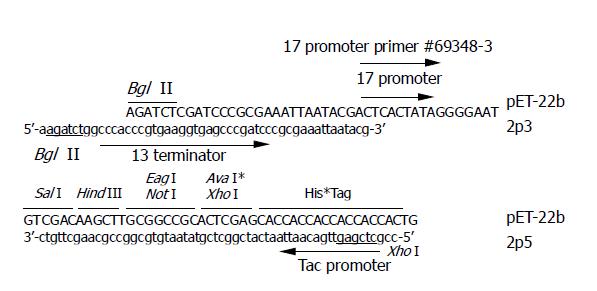
A 291-bp DNA fragment was amplified from pET-22b plasmid DNA (Novagen, USA) as template. The primers are 2p5 5’-ccgctcgagttgacaattaatcatcggctcgtataa-tgtgcggccgcaagcttgtc-3’ and 2p3 5’-aagatctggcccacccgtgaaggtgagcccgatcccgcgaaattaatacg-3’ (Figure 1). The amplified fragment was digested with restriction enzyme Bgl II and Xho I and cloned into pET-22b at the corresponding sites. The obtained plasmid (named pET-2P) was verified by sequencing and used as dsRNA expressing vector.
Figure 1 Sequences of primer 2p5 and 2p3.
The last 18 nt of 2p5 correspond to the antisense strand before Xho I site of pET-22b, the nucleotide sequence in gray box of 2p5 correspond to the tac promoter, and the underlined letters represent the Xho I site. The last 21 nt of 2p3 correspond to the sense strand after the Bgl I site of pET-22b, the nucleotide sequence in gray box of 2p3 correspond to the T3 terminator, and the underlined letters represent the Bgl II site.
A DNA fragment representing part of HBV polymerase (designated HBVP) was amplified from plasmid pUC-HBV2 using the following oligos: 5’-ggaattcgtcttgggtatacatttgacc-3’ (EcoR I site underlined) and 5’-ggggtaccagaggacaacagagttg-3’ (Kpn I site underlined). A 422-bp DNA fragment representing part of alkaline phosphatase was amplified from pSEAP2-control plasmid DNA using the following oligos: 5’-ggaattcagctgccaggatcctaaaag-3’ (EcoR I site underlined) and 5’-agctgtcgacatgagctgcgtagcgatgtc-3’ (Sal I site underlined). The above DNA fragments were first cloned into pUC118 between the introduced restriction enzyme recognition sites. The two DNA fragments were then inserted into pET-2P between EcoR I and Sal I sites. The obtained plasmids were named pET-HBVP and pET-AP, respectively.
The EcoR I/Not I fragment of the pEGFP-N2 plasmid was cloned into pET-2P at the corresponding sites and the resulting plasmid was used to express dsRNA of GFP.
Construction of HBsAg expression plasmid
The 451-bp DNA fragment at the 5’ end of HBV large S coding sequence was amplified from the pUC-HBV2 plasmid DNA using oligos 5’-cgggatccatgggaggttggtct-tccaaac-3’ (BamH I site underlined) and 5’-ggaatcctgatgtt-gtgttctc-3’. The fragment was digested with BamH I and Xho I and inserted into pEGFP-N2 (named pEGFP-HBs5) between Bgl II and Xho I sites. A 1.3 Kb Xho I/BamH I fragment of pUC-HBV2 plasmid DNA was cloned into pEGFP-HBs5 between Xho I and BamH I sites. Thus the obtained plasmid (named pEGFP-HBS) contains the full length of the coding sequence of large S antigen and an additional HBVP fragment.
Expression and purification of dsRNAs
The above obtained dsRNA expression vectors were transformed into E.coli strain BL21(DE3). The transformants were inoculated into LB broth supplemented with 100 μg/mL ampicillin and cultured at 37 °C overnight with shaking (250 r/min). Ten milliliters of seed culture was transferred into 1 L fresh medium and cultured at the same condition until A600 reached 1.0 when IPTG was added to the final concentration of 0.25 mmol/L. The bacterial cells were cultured for another 3 h before collection by centrifugation. Nucleic acid extraction was performed according to the protocol of small-scale preparation of plasmid DNA[27] with modification. The aqueous phase of phenol/chloroform extraction was collected and 1/5 th volume of 100% ethanol was added before loading onto CF 11 column equilibrated with 1X STE (10 mmol/L Tris-HCl, 100 mmol/L NaCl, 1 mmol/L EDTA, pH 8.0) containing 20% ethanol. Single stranded RNA and DNA was washed off the column with five column volumes of 1XSTE containing 18% ethanol. The dsRNA was then eluted with 20 mL 1XSTE and precipitated by 2 volumes of 100% ethanol. The RNA pellet was washed with 75% ethanol once and air-dried pellet was resuspended in water. The concentration of dsRNA preparation was measured with absorbance at 260 nm.
siRNA preparation
E. coli RNase III gene was amplified from the E. coli genomic DNA with the following primers: 5’-ggaattcatgaaccccatcg-taattaatcg-3’ (EcoR I) and 5’-ggggtacctcattccagctccagttttttc-3’ (Kpn I). The 681-bp DNA fragment was cloned into pET-30a to obtain RNase III expressing plasmid pET-RNaseIII which then transformed into BL21 (DE3). The transformed cells were cultured at 37 °C in LB medium supplemented with 25 µg/mL kanamycin and induced by 100 µmol/L IPTG for 4 h. The cells were collected by centrifugation at 4000 g for 10 min at 4 °C and resuspended in 40 mL lysis buffer (50 mmol/L Tris-HCl, 250 mmol/L NaCl, 10 mmol/L dithiothreitol, 4 mg/mL lysozyme, pH 8.0) per liter of original culture medium. The suspension was incubated at 4 °C for an hour and further treated with sonication. The cell debris was removed by centrifugation at 10000 g, 4 °C for 15 min, and the supernatant was loaded onto Ni-NTA His•Bind Resin and the protein purification was done according to manufacturer’s recommendation. The recombinant RNase III was eluted with 20 mmol/L Tris-HCl, 100 mmol/L NaCl, 150 mmol/L imidazole (pH 8.0). The eluate was dialyzed against 50 mmol/L Tris-HCl, 50 mmol/L NaCl and 50% glycerol (pH 7.5).
For preparation of siRNAs, 100 μg purified dsRNA was digested with 100 μg recombinant RNase III in 400 μL reaction mixture containing 50 mmol/L Tris-HCl, 50 mmol/L NaCl, 10 mmol/L MnCl2 and 1 mmol/L DTT (pH 7.5) at 37 °C for 1 h. The reaction mixture was separated on 15% non-denaturing polyacrylamide gel, and the siRNAs about 25 bp in length was recovered with the method described by Yang et al[23].
Cell culture and transfection
SMMC-7721 cells and 293T cells were maintained in RPMI 1640 medium supplemented with 100 mL/L fetal calf serum (HYCLONE, USA), 100 μg/mL streptomycin and 100 IU/mL penicillin at 37 °C in a humidified atmosphere containing 50 mL/L CO2. HepG2 cells were maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) supplemented with 100 mL/L serum, 100 μg/mL streptomycin and 100 IU/mL penicillin. All cells were seeded into 24-well plates, 24 h prior to transfection. For all transfections, appropriate amount of plasmid DNA and siRNA were delivered into the cells using Lipofectamine 2000 according to the manufacturer’s instructions.
Enzyme activity assay of secreted alkaline phosphatase
Forty microliters of supernatant of culture medium was added into 160 μL enzyme assay buffer (0.1 mol/L Tris-HCl, 0.1 mol/L NaCl, 1 mmol/L MgCl2, pH 9.5) containing 20 mmol/L pNPP (Sigma 104 Phosphatase substrate number 104-100) in a 96-well microtiter plate, then incubated at 37 °C for 15 min. The absorbance at 405 nm was measured.
Measurement of HBsAg and HBeAg expression levels
HBsAg and HBeAg expression levels were measured by Diagnostic kits for hepatitis B surface antigen and e antigen following the instructions of the provider.
RESULTS
dsRNA production
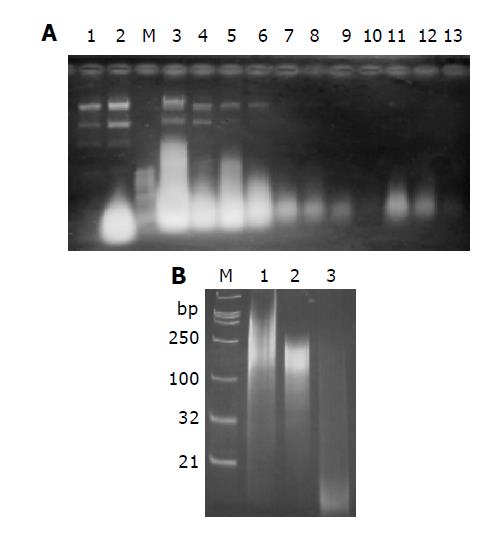
The dsRNA expression vectors were transformed into E.coli strain BL21 (DE3) and the dsRNA was expressed and purified as described in materials and methods. As shown in Figure 2A, plasmid DNA and single stranded RNA could be washed off with 1XSTE containing 18% ethanol. The dsRNA was eluted with 1XSTE. The purified RNA could be cleaved into 12-15 bp long oligonucleotides by dsRNA processing enzyme E.coli RNase III (Figure 2B, Lane 3), while it remained nearly intact when treated with ssRNA preferring bovine pancreatic RNase A (Figure 2B, Lane 2). However, trivial changing of the band pattern was detected after RNase A treatment. Probably RNase A treatment cleaved off the single stranded extension of the end of the purified dsRNAs. Nonetheless, the data confirmed that the RNAs purified here were dsRNAs. According to the polyacrylamide gel electrophoresis, the RNAs prepared in this manner were smaller than their corresponding DNA insert, and several bands of different molecular weight were detected (Figure 2B, Lanes 1 and 2). The reason behind this phenomenon is not clear, but it is possible that dsRNAs were immediately degraded by endogenous RNase III to some degree. The productivity of dsRNAs varied for genes with an average of approximately 4 mg/L of original culture.
Figure 2 RNAs analysis on 1% agarose gel (A) and 15% non-denaturing polyacrylamide gel (B).
A: RNAs extracted from cells without induction (Lane 1) and with induction with IPTG (Lane 2). The highlight spot represents dsRNAs. dsRNA was purified with CF 11 column as described in materials and methods. Lane 3: RNA extract sample loaded onto CF 11 column; lane 4: flow through; lane 5-10: wash-off with STE containing 18% ethanol (mostly plasmid DNA and single stranded RNA); Lane 11-13: eluate with STE; M: 1 Kb DNA ladder as Marker; B: Digestion analysis of purified dsRNA with RNase A or RNase III. Lane 1: 1 μg undigested purified dsRNA; lane 2: digestion with 0.1 μg RNase A for an hour at 37 °C; lane 3: digestion with 1 U recombinant E. coli RNase III (Ambion) for an hour at 37 °C; M: DNA marker. The length of marker is indicated on the left.
Preparation of recombinant RNase III and siRNAs
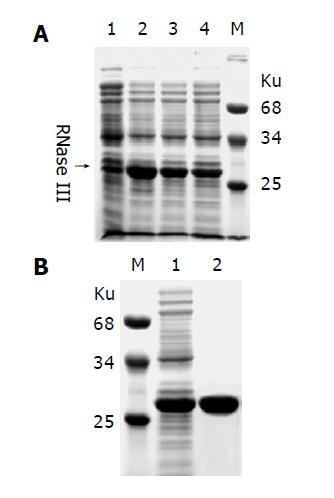
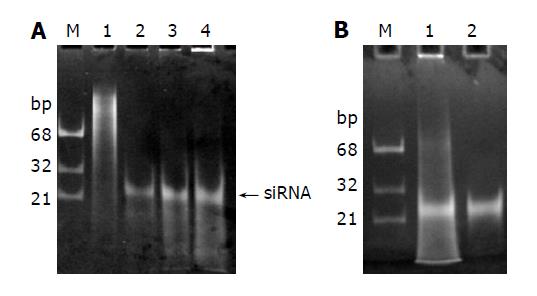
In order to prepare siRNAs, we first produced and purified recombinant E. coli RNase III as described in materials and methods. As shown in Figure 3A, high expression level was obtained and the recombinant RNase III can be purified by a single step affinity chromatography with Ni-NTA His•Bind resin. E.coli RNase III is a dsRNA specific endonuclease, known to digest long dsRNAs into short fragments about 12-15 bp in length, which are too small to trigger RNAi. However, when magnesium ions were replaced with manganese ions, dsRNAs could be processed by RNase III into about 25 bp long oligonucleotides (Figure 4A), which were purified from polyacrylamide gel as described by Yang et al[23] and considered as siRNAs for further use.
Figure 3 Expression and purification of recombinant E.
coli RNase III. A: E. coli RNase III was expressed as an N-terminal His-tagged fusion protein in E. coli strain BL21(DE3). Lane 1: negative control; lanes 2-4 represent 3 individual clones induced with IPTG; B: The recombinant protein extract (Lane 1) was purified by a single step affinity chromatography with the Ni-NTA His•Bind Resin (Lane 2). Molecular weight of marker proteins is indicated nearby.
Figure 4 Processing of long dsRNAs into siRNAs with recombinant E.
coli RNase III. A: 1, 3 and 5 μg dsRNAs (Lanes 2-4) were digested with 1 µg RNase III at 37 °C for 1 h in 10 µg reaction mixture containing 50 mmol/l Tris–HCl, 50 mmol/L NaCl, 10 mmol/L MnCl2 and 1 mmol/L DTT (pH 7.5). The siRNA-like products were indicated by an arrow on the right. Lane 1: undigested dsRNAs; B: The siRNAs were recovered from the gel (Lane 2).
Inhibition of GFP expression in SMMC7721 cells
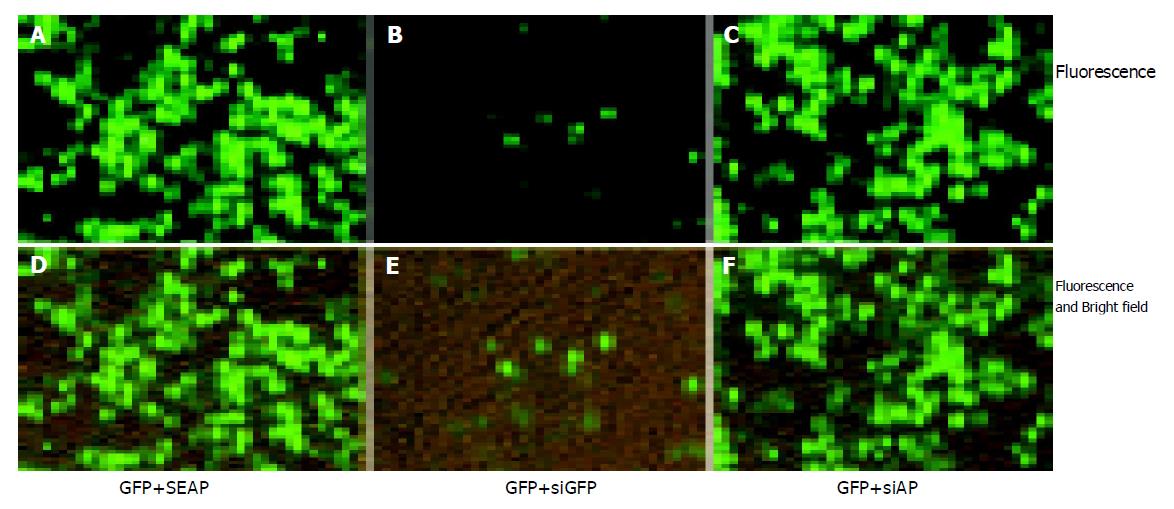
In order to test whether the siRNAs prepared with CFDP can be used to induce RNA interference in mammalian cells, we prepared siRNAs corresponding to GFP coding sequence (siGFP) and SEAP coding sequence (siAP). siRNAs were co-transfected into SMMC7721 cells with pEGFP-N2 plasmid. As shown in Figure 5A, co-transfection of siGFP remarkably reduced the GFP expression level from pEGFP-N2 (Figure 5B, Figure E), while co-transfection of siAP did not (Figure 5C, Figure F). Similar result was obtained with the 293T cells (data not shown). The result proved siRNAs prepared with CFDP can dramatically and specifically inhibit homologous gene expression.
Figure 5 Specific inhibition of GFP expression in SMMC7721 cells.
0.4 μg pEGFP-N2 plasmid DNA were co-transfected with 0.4 μg pSEAP2-control plasmid DNA (A, D) or 0.4 μg siGFP (B, E), or 0.4 μg siAP (C, F) into the SMMC7721 cells in a 24-well plate. Cells were photographed 48 h post transfection. 0.4 μg pSEAP2-control plasmid was added in control group to ensure that transfection parameters remain the same. The total amount of nucleic acids was 0.8 μg for each group and the package efficiency of siRNAs and plasmid DNAs with lipofectamine 2000 was assumed to be equal. The fluorescent and bright-field pictures (D–F) show that the cell density was the same for all groups. Three independent experiments were performed, and one typical picture was shown.
Inhibition of HBsAg expression in SMMC7721 cells
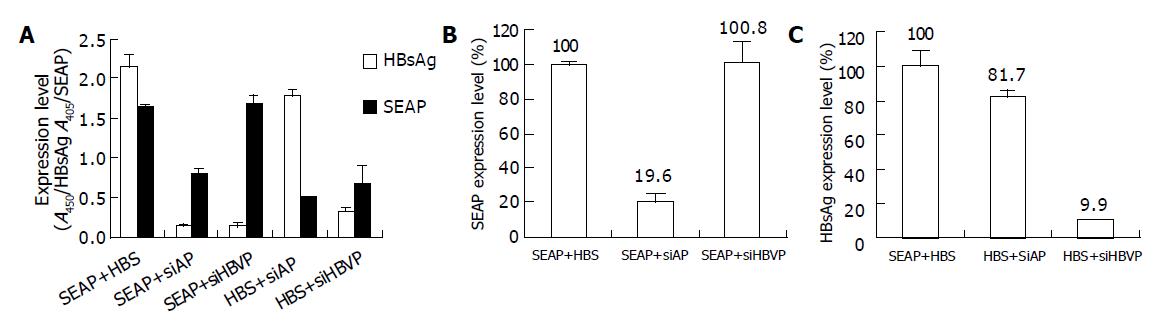
We then tested the feasibility of inhibition of HBsAg expression in SMMC7721 cells, a human liver cancer cell line known to possess high transfection efficiency. siRNAs corresponding to part of HBV polymerase coding sequence (siHBVP) were prepared. As shown in Figure 6, siHBVP specifically inhibited the HBsAg expression but did not inhibit SEAP expression, while siAP specifically inhibited SEAP expression with a little reduction of HBsAg expression. The HBsAg expression was reduced to 10% of the control when co-transfected with 0.4 μg siHBVP (Figure 6C). The final concentration of the siRNAs was about 60 nmol/L. Remarkable inhibition rate was obtained at low siRNA concentration.
Figure 6 Inhibition of HBsAg expression in SMMC7721 cells.
SMMC7721 cells were co-transfected with 0.4 μg siHBVP and 0.4 μg pEGFP-HBS (HBS+siHBVP) or 0.4 μg pSEAP2-control (SEAP+siHBVP). As control cells were transfected with 0.4 μg siAP and 0.4 μg pEGFP-HBS (HBS+siAP) or 0.4 μg pSEAP2-control (SEAP+siAP). The positive control group was co-transfected with 0.4 μg pEGFP-HBS and 0.4 μg pSEAP2-control (SEAP+HBS). 48 h post transfection. The supernatants were collected for measuring expression level of secreted alkaline phosphatase, and the cells were lysed with RIPA Buffer for measuring HBsAg expression. A: shows the SEAP and HBsAg expression level represented by OD values; B, C: show the HBsAg or SEAP expression level relative to the positive control. Values represent averages of three independent experiments, with the error bars indicating the standard deviation.
Inhibition of HBsAg and HBeAg expression in HepG2 cells
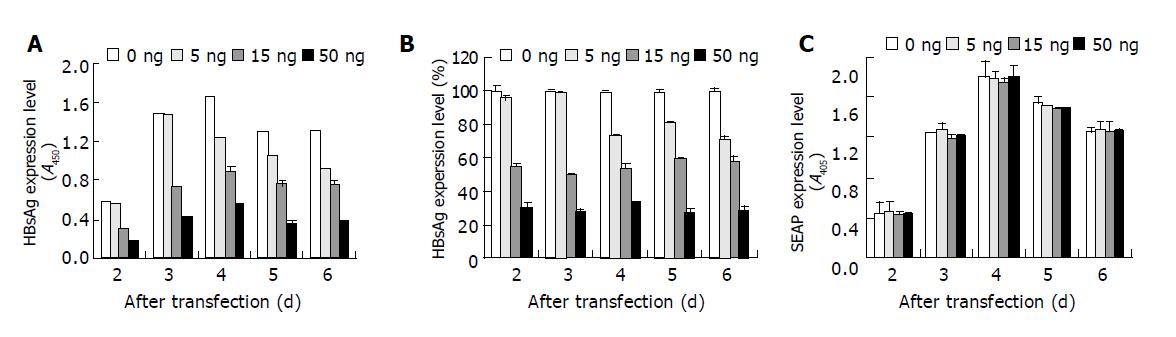
The dose-dependent effect of siHBVP was further tested in HepG2 cells. Much lower siHBVP concentration was used to observe the dose-dependent effect. 0, 5, 15 or 50 ng siHBVP was co-transfected with 0.35 μg pUC-HBV2 and 0.35 μg pSEAP2-Control into HepG2 cells. The culture media were collected every 24 h and fresh media were added. The expression level of HBsAg, HBeAg and alkaline phosphatase secreted into the culture media were measured. As shown in Figure 7, HBsAg expression was inhibited by siHBVP, and a dose-dependent effect was clearly detected (Figure 7B). At the same time, the expression of control gene (SEAP) was not inhibited (Figure 7C). The inhibition rate remained at the same level during 6 d after transfection, although the expression level was changed (Figure 7A). A dose-dependent inhibition of HBeAg expression was also detected (Data not shown).
Figure 7 Dose dependent inhibition of HBsAg expression in HepG2 cells.
0, 5, 15 or 50 ng siHBVP was co-transfected with 0.35 μg pUC-HBV2 and 0.35 μg pSEAP2-control into HepG2 cells. The supernatant of culture media were removed every 24 h and fresh media were added. The HBsAg and alkaline phosphatase secreted into the culture media were measured. A and C show the HBsAg or SEAP expression level represented by OD values. B shows the HBsAg expression level relative to the positive control. Values represent averages of three independent experiments, with the error bars indicating the standard deviation.
DISCUSSION
Because of its high efficiency and specificity, great efforts have been devoted to applying RNAi to disease treatment, especially in cancer therapy and anti-virus infection[28-31]. Although successes have been made in cell culture and animal studies, there are still many obstacles to be overcome. Only synthesized siRNAs or siRNAs produced by in vitro transcription and subsequent enzymatic digestion have been used in mammalian cell systems so far. In this paper, we describe a novel cost-effective siRNA preparation method. We showed that dsRNAs can be easily prepared by fermentation in E.coli and purified by affinity chromatography. We also show dsRNAs produced this way can be easily processed into siRNA-like products by recombinant E.coli RNase III, which can remarkably and specifically inhibit the expression of target gene in mammalian cells. HBsAg expression level was reduced to 10% in SMMC7721 cells when 60 nmol/L of siHBVP was used. Its efficiency and specificity are comparable to that of siRNAs prepared by other methods. A dose-dependent inhibition effect was detected in HepG2 cells co-transfected with HBV replication competent plasmid and different amount of siHBVP. The HBsAg secretion was inhibited by 70% when 7.5 nmol/L siHBVP was used. Its inhibition effect remained at least 6 d after transfection. The lower inhibition rate in HepG2 cells (70% compared with 90% obtained in SMMC7721 cells) was because of the lower siRNA concentration used in HepG2 cells. There are two reasons for the use of siRNA of lower concentration. One is that the dose-dependent effect can only be observed at lower siRNA concentrations, the other is that the transfection parameters (N/P) can be kept the same for different groups only when lower siRNA concentration is used, which is critical in keeping transfection efficiency as the same. One possible problem is that the siRNAs prepared by this method is a mixture of heterogeneous sequences, which may increase the risk of off-target gene suppression caused by sequence similarity. However, we did not observe severe off-target gene suppression. In fact, the sequence diversity of the siRNA mixture may be helpful to overcome the problem of drug resistance caused by the rapid mutation of virus genome. In conclusion, the novel siRNA preparation method makes it possible to produce siRNAs on a large scale at a relatively low cost, and the method will find applications in anti-virus research and therapy.