Published online Feb 28, 2005. doi: 10.3748/wjg.v11.i8.1210
Revised: June 18, 2004
Accepted: July 22, 2004
Published online: February 28, 2005
AIM: To determine whether the mast cell (MCs) and tumor-associated macrophage (TAMs) counts have any correlation with clinical outcome in colorectal cancer, and to investigate whether MCs undergo phenotypic changes in colorectal cancer.
METHODS: The MC and TAM counts were determined immunohistochemically in 60 patients with colorectal cancer and the depth of invasion, lymph node metastasis rate, distant metastasis rates, and survival rates were compared between patients with low (less than the mean number of positive cells) and high (more than the mean number of positive cells) cell counts.
RESULTS: Both patients with a low MC count and patients with a low TAM count had significantly deeper depth of invasion than those with a high MC count and those with a high TAM count (P<0.01 and P<0.01 respectively). Patients with a high MC count and patients with a high TAM count were significantly higher showing significantly lower rates of lymph node metastasis, distant metastasis than those with a low MC count and those with a low TAM count. There were significant positive correlation between MC counts and TAM counts (r = 0.852, P<0.01). In both cancerous tissue and normal colorectal tissue, the predominant MC phenotype was MCTC. The 5-year survival rate estimated was significantly lower in both patients with a low MC count and patients with a low TAM count than in those with a high MC count and those with a high TAM count (P<0.05 and P<0.01 respectively).
CONCLUSION: There appears to be a direct relationship between the number of MCs and clinical outcome in patients with colorectal cancer, even though MCs exhibited no significant phenotypic changes. TAM count is of value to predict the clinical outcome or prognosis. It is more beneficial for estimating biological character of colorectal carcinoma to combine MC and TAM counts.
- Citation: Tan SY, Fan Y, Luo HS, Shen ZX, Guo Y, Zhao LJ. Prognostic significance of cell infiltrations of immunosurveillance in colorectal cancer. World J Gastroenterol 2005; 11(8): 1210-1214
- URL: https://www.wjgnet.com/1007-9327/full/v11/i8/1210.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i8.1210
Numerous cells of immunosurveillance are known to microscopically infiltrate in cancers. These infiltrating immunosurveillance cells, including mast cell (MCs) and tumor associated macrophages (TAMs), have been found to reflect a tumor-related immune response[1-4].
In the fight against cancer, MCs have been shown to play several roles. While some studies report on the antitumor functions of MCs including natural cytotoxicity[1] and the release of antitumor compounds[2], in addition to the enhancement of the cytotoxic activation of mainly peritumoral eosinophils and macrophages[5-8], others suggest a direct relationship between MCs and tumor angiogenesis[9,10]. Many theories have been advanced as to the reasons for the antitumor functions of MCs, in particular as to whether phenotypic changes occur in the presence of cancer cells[11-13]. It is well known that human MCs are conventionally divided into two types depending on the expression of different proteases in their granules: MCs positive only for tryptase (MCT) and MCs positive for both tryptase and chymase (MCTC). Phenotypic changes have been reported in pathological conditions including helminth infections, sarcoidosis and allergic alveolitis[14,15]. Unlike the definitive studies already mentioned, however, few researches have demonstrated a clear correlation between MCs and tumor toxicity in colorectal cancer, and, as yet, the MC phenotype in lung cancer has not been characterized.
Although TAMs have the potential to mediate tumor cytotoxicity and to stimulate antitumor lymphocytes[3], it is still unclear whether TAMs can predict tumor prognosis.
The purpose of this study was to determine: (1) whether a direct correlation exists between the number of these inflammatory cells (MCs and TAMs) and the clinical outcome of patients with colorectal cancer, and (2) whether these MCs undergo phenotypic changes in colorectal cancer.
A total of 60 carcinomas of the colon and rectum (35 men and 25 women, mean age: 67.9 years, range 48-80 years) were selected from our department and affiliated hospitals between 1995 and 2002. We also obtained 20 “normal” colon and rectum specimens (mean age = 59 years, age range 41-86 years, male:female = 12:8) from the biopsy and autopsy files of our department. Depth of invasion was graded according to the standard TNM classification.
All specimens were immediately fixed in neutral-buffered formalin and embedded in paraffin wax. Several 4-μm serial sections were obtained from each case. They were subjected to immunohistochemical analysis.
Mast cell immunohistochemical staining and estimation of mast cell phenotype[19] To distinguish MCT and MCTC, two serial sections were stained for both chymase and tryptase, both of which were known to be contained in the granules of MC cytoplasm, using the EnVision method and the streptavidin-peroxidase conjugated method (S-P) methods[14].
After deparaffinization and rehydration, for antigen retrieval the sections were microwaved at 750 W for 10 min. the specimens were incubated with 3% H202, for 10 min to quench endogenous peroxidase activity, and incubated with 1.5% non-immune goat serum for 20 min to suppress nonspecific binding of subsequent reagents. Then they were incubated with two primary antibodies, namely primary antibody CC1 (monoclonal mouse anti-human MC chymase, Novocastra, dilution = 1:500), and primary antibody AA1 (monoclonal mouse anti-human MC tryptase, Novocastra, dilution = 1:1000) for 60 min at 25 °C. After washing with PBS, the slides were subsequently incubated respectively with Polymer Helper (PV9000 kits, Zhongshan Bio Corp., Beijing, China) for 20 min and biotin-conjugated goat anti-mouse IgG antibody for 15 min. Then, after washing again with PBS, the sections were incubated respectively with poly peroxidase-goat-anti-mouse IgG antibody for 30 min and streptavidin-peroxidase complex (SP kits, Zhongshan Bio Corp., Beijing, China) for 15 min. Diaminobenzidine was used as chromogen to yield brown reaction products. Nuclei were counterstained with Harris hematoxylin.
Using light microscopy, the mean number of chymase-positive MCs/total MCs per 5 fields with the most abundant infiltration at a magnification of 400× was counted, and the ratio was calculated. Chymase-positive MCs were considered to be MCTC, all others, MCT.
After deparaffinization, for antigen retrieval, the sections were microwaved at 750 W for 10 min and the sections were incubated in 3% hydrogen peroxide for 10 min in order to devitalize endogenous peroxidase. Deparaffinized and rehydrated specimens were heated in 10 mmol/L citrate buffer, pH 6.0, for 10 min in an autoclave at 120 °C. After cooling to room temperature for 30 min, the specimens were incubated with normal goat serum for 15 min at 37 °C. Then they were incubated with monoclonal antibody against CD68 (Zhongshan Bio Corp.) for 60 min at 37 °C, followed by the S-P technique using an S-P kit (Zhongshan Bio Corp., Beijing, China) and diaminobenzidine as the chromogen. Nuclei were counterstained with Harris hematoxylin.
The mean number of TAMs per 5 fields with the most abundant infiltration at a magnification of 400× was counted.
Correlation between the degree of infiltration by each cell type and the various clinicopathologic factors was analyzed using the Student’s t-test, Mann-Whitney U test, χ2 test, and Pearson correlation was performed for correlation analysis between MCs and TAMs. Survival curves were constructed using the Kaplan-Meier method and differences were tested using log-rank statistics. The level of critical significance was assigned at P<0.05.
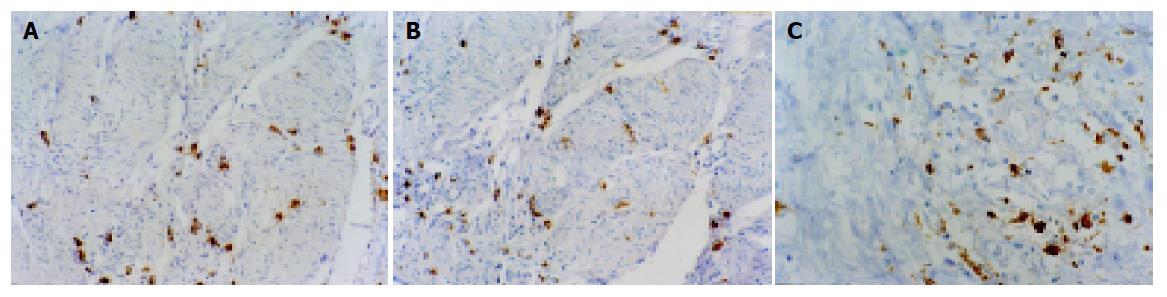
Immunohistochemical staining indicated that tryptase, chymase and CD68 were localized in the cytoplasm. Infiltrating MCs and TAMs were distributed primarily in the invasive area of the cancers (Figure 1). At ×400 magnification, the mean number of total MCs was 59.2, and of TAM 81.2. Of the 60 patients, 27 had 59 or more MCs and were ascribed to the high level of MCs infiltration group, while the remaining 33 patients had 58 or less MCs and were put into the low level of MCs infiltration group. Twenty-nine patients had 81 or more TAMs and were denoted by the high level of TAM infiltration group, while the remaining 31 patients had 80 or less TAMs and were denoted by the low level of TAM infiltration group.
Table 1 shows the correlation between the expression of each antigen and the various clinicopathologic factors. Patients with a low level of MC infiltration had significantly deeper depth of invasion than those with a high level of MC infiltration (P<0.01). The percentage of patients with a high level of MC infiltration was significantly higher in lymph node metastasis-negative cases (59.3%) than those with a low MC count in lymph node metastasis-negative cases (27.3%, P<0.05), and higher in distant metastasis-negative cases (74.1%) than in distant metastasis-negative cases (48.5%, P<0.05).
| MCs | TAMs | |||
| High1 | Low2 | High3 | Low4 | |
| Age (yr)5 Sex7 | 61.2 | 57.2 | 58.3 | 59.7 |
| Male | 13 | 22 | 16 | 19 |
| Female | 14 | 11 | 13 | 12 |
| Histological grade of carcinoma6 | ||||
| Well | 12 | 9 | 14 | 10 |
| Moderate | 11 | 14 | 12 | 12 |
| Poor | 4 | 10 | 3 | 9 |
| Depth of invasion7 | ||||
| Superficial Ta-T1 | 19b | 10 | 19d | 9 |
| Invasive T2-T4 | 8b | 23 | 10d | 22 |
| Lymph node metastasis7 | ||||
| Positive | 11a | 24 | 12c | 22 |
| Negative | 16a | 9 | 17c | 9 |
| Distant metastasis7 | ||||
| Positive | 7a | 17 | 8c | 18 |
| Negative | 20a | 16 | 21c | 13 |
Patients with a low TAM count had significantly deeper depth of invasion than those with a high TAM count (P<0.01). The percentage of patients with a low TAM count was significantly higher in lymph node metastasis-positive cases (71.0%) than those with a high TAM count in lymph node metastasis-positive cases (41.4%, P<0.05), and in lymph node metastasis-positive cases (58.1%) than in lymph node metastasis-positive cases (27.6%, P<0.05).
With regard to mast cell subpopulations, MCTC always predominated over MCT in each group. The percentage of MCT and MCTC in colorectal cancer group and normal group is shown in Table 2. The percentage of MCT was 22.6%, and that of MCTC was 77.4% in colorectal cancer. Although the quantities of MCT and MCTC were significantly higher in the tissue of colorectal carcinoma than in normal colorectal tissue, there were no significant differences in this percentage between normal groups and colorectal cancer groups (Table 2).
| MCTC (mean±SD) | MCT (mean±SD) | Total | |
| Normal group (n = 20) | 10.13.2 (74.3%) | 3.50.9 (25.7%) | 13.62.1 (100%) |
| Colorectal cancer group (n=60) | 45.810.6 (77.4%) | 13.44.9 (22.6%) | 59.29.3 (100%) |
The percentage of MCT and MCTC in colorectal cancer with various histological grades was all approximately 25% and 75% respectively. There were no differences in the percentage of MCT and MCTC among well, moderately and poorly differentiated tumors (Table 3).
| Histological grade of carcinoma | MCTC (mean±SD) | MCT (mean±SD) | Total |
| Well (n = 21) | 50.812.5 (80.5%) | 12.35.1 (19.5%) | 63.110.1 (100%) |
| Moderate (n = 25) | 42.29.1 (73.0%) | 15.63.3 (27.0%) | 57.88.2 (100%) |
| Poor (n = 14) | 44.78.5 (80.0%) | 11.24.7 (20.0%) | 55.95.3 (100%) |
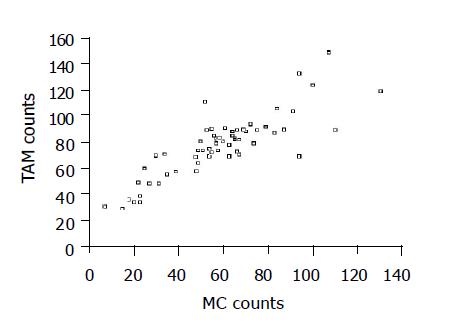
There was significant positive correlation between MC and TAM counts (Figure 2. r = 0.852, P<0.0001).
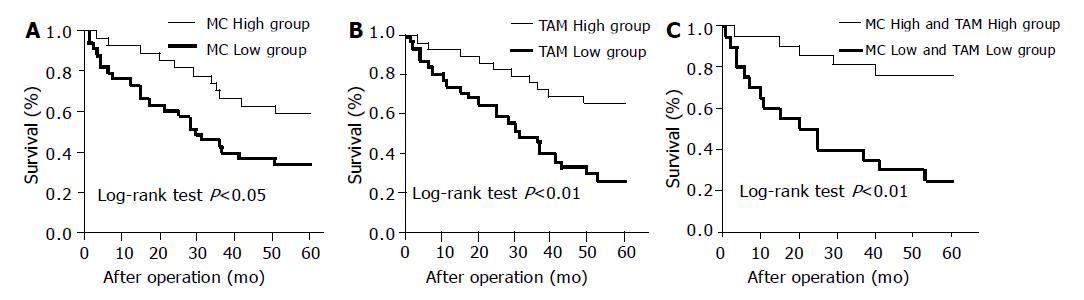
Survival at 5 years was 59.3% in patients with a high MC count compared to 33.3% in those patients with a low MC count. Overall survival was significantly shorter for patients with a low MC count than those with a high MC count (Figure 3A, log-rank test, P<0.01).
Survival at 5 years was 65.5% in patients with a high TAM count and 25.8% in patients with a low TAM count. There is a significant difference observed between the two groups (Figure 3B, log-rank test, P<0.01).
Twenty-one patients had a high level of both MC and TAM infiltrations. This group’s survival rate at 5 years (76.2%) was higher than the patients with a low level of both MC and TAM infiltrations (25.0%, Figure 3C).
In this study, we evaluated the relationship between MCs and the clinical outcome in 60 randomly selected patients with colorectal cancer, and found a significant correlation between MC count and patient prognosis. Similar observations have been reported in lung cancer[16] as well as breast cancer[17]. These results suggest that MCs may exert a cytotoxic effect on cancer cells.
The effector components in the MC granules that suppress tumor cell activity have not been clearly identified. MCs contain the serine proteases, tryptase and chymase[18]. One study indicates that MC proteases may kill tumor cells directly by destroying surface structures on tumor cells and indirectly by altering the medium in a fashion analogous to the effect of arginase[19].
In addition to these effector components, experimental studies have shown that MC chymase induces the accumulation of macrophages, neutrophils and other inflammatory cells in vivo[8]. Furthermore, MC enhances the cytotoxic activation of mainly peritumoral macrophages and eosinophils[5-8] and may indirectly exert a cytotoxic effect on cancer cells. These suggestions that MCs may inhibit the malignant progression of carcinomas are supported in our study by the higher number of MCs in High Group, which had a better prognosis than Low Group, which had fewer MCs.
Other studies, however, have reported on the relationship between MCs and tumor angiogenesis[9,10,20]. Secreting MCs are able to induce and enhance angiogenesis via multiple, in part, interacting pathways[20]. As has been demonstrated, MC secreting products such as basic fibroblast growth factor[21] and histamine[22] may act directly on endothelial cells by stimulating their migration and proliferation. VEGF, a well-demonstrated mediator secreted by MCs, may contribute to angiogenesis[23]. Recently, MC tryptase was found to be a novel and potent angiogenic factor[24]. On account of this information, MCs may be seen to play a role in promoting tumor growth. However, our results, in addition to other studies, indicate that MCs are cytotoxic to cancer cells. Because of these conflicting reports, further studies are required to ascertain whether MCs enhance host immunity against cancer cells or accelerate tumor growth.
Several studies have focused on MC phenotypes in order to identify the effector components of MCs and their particular roles. Because phenotypic changes have been reported in pathological conditions including helminth infections, sarcoidosis and allergic alveolitis[14,15], we were interested in determining whether MCs might undergo phenotypic changes in colorectal cancer. The present study revealed: while there was significant difference in the quantities of MCT and MCTC between colorectal cancer and normal colorectal tissue, no difference was found in the proportion of MCT and MCTC between colorectal cancer and normal colorectal tissues. This finding suggests that both MCT and MCTC may equally proliferate or infiltrate in colorectal cancer.
It is well known that TAMs have numerous functions and, when activated, TAMs inhibit cancer growth and destroy cancer cells[25]. To date, a significant difference in the degree of TAM infiltration has been reported with regard to human bladder cancer[26]. However, whether correlation exists between the degree of TAM infiltration and cancer progression and prognosis remains unclear. Our results demonstrate that TAMs may exert a suppressive function against cancer progression and occurrence of regional lymph node metastasis and distant metastasis. Furthermore, a high level of TAM infiltration had some impact on prognosis. Therefore, TAM infiltration may manifest sufficient systemic anti-tumoral effect.
In the present study, there was significant positive correlation between MC counts and TAM counts, and 16 patients with a high level of both MC and TAM infiltration (76.2%) survived for 5 years after surgery. MC secreting product-chymase induces the accumulation of macrophages, neutrophils and other inflammatory cells in vivo[8]. Furthermore, MC also enhances the cytotoxic activation of TAMs and eosinophils[5-8]. Our findings support those results by showing that the cooperative interaction of TAMs and MCs is important for anti-tumoral immunoreaction.
In conclusion, MC and TAM count were found to have a direct relationship with clinical outcome in patients with colorectal cancer. From these results, we presume that MCs and TAMs may play an important role in the enhancement of host immunity against cancer cells and that an increase in MCs and TAMs may improve the postoperative prognosis of patients with colorectal cancer. It is beneficial for estimating biological character of colorectal carcinoma to combine MC and TAM counts. Further studies are required with respect to MC phenotyping to confirm whether or not changes do indeed occur in the presence of colorectal cancer.
Co-first-authors: Shi-Yun Tan and Yan Fan
| 1. | Ghiara P, Boraschi D, Villa L, Scapigliati G, Taddei C, Tagliabue A. In vitro generated mast cells express natural cytotoxicity against tumour cells. Immunology. 1985;55:317-324. [PubMed] |
| 2. | Henderson WR, Chi EY, Jong EC, Klebanoff SJ. Mast cell-mediated tumor-cell cytotoxicity. Role of the peroxidase system. J Exp Med. 1981;153:520-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Alleva DG, Askew D, Burger CJ, Elgert KD. Fibrosarcoma-induced increase in macrophage tumor necrosis factor alpha synthesis suppresses T cell responses. J Leukoc Biol. 1993;54:152-160. [PubMed] |
| 4. | Tani K, Ogushi F, Shimizu T, Sone S. Protease-induced leukocyte chemotaxis and activation: roles in host defense and inflammation. J Med Invest. 2001;48:133-141. [PubMed] |
| 5. | Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23-F26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 282] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Dimitriadou V, Koutsilieris M. Mast cell-tumor cell interactions: for or against tumour growth and metastasis? Anticancer Res. 1997;17:1541-1549. [PubMed] |
| 8. | He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol. 1998;125:1491-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Roche WR. Mast cells and tumors. The specific enhancement of tumor proliferation in vitro. Am J Pathol. 1985;119:57-64. [PubMed] |
| 10. | Marks RM, Roche WR, Czerniecki M, Penny R, Nelson DS. Mast cell granules cause proliferation of human microvascular endothelial cells. Lab Invest. 1986;55:289-294. [PubMed] |
| 11. | Matsunaga Y, Terada T. Mast cell subpopulations in chronic inflammatory hepatobiliary diseases. Liver. 2000;20:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. 2000;33:961-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Nagata M, Shijubo N, Walls AF, Ichimiya S, Abe S, Sato N. Chymase-positive mast cells in small sized adenocarcinoma of the lung. Virchows Arch. 2003;443:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Arizono N, Koreto O, Nakao S, Iwai Y, Kushima R, Takeoka O. Phenotypic changes in mast cells proliferating in the rat lung following infection with Nippostrongylus brasiliensis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Walls AF, Roberts JA, Godfrey RC, Church MK, Holgate ST. Histochemical heterogeneity of human mast cells: disease-related differences in mast cell subsets recovered by bronchoalveolar lavage. Int Arch Allergy Appl Immunol. 1990;92:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Tomita M, Matsuzaki Y, Onitsuka T. Correlation between mast cells and survival rates in patients with pulmonary adenocarcinoma. Lung Cancer. 1999;26:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Aaltomaa S, Lipponen P, Papinaho S, Kosma VM. Mast cells in breast cancer. Anticancer Res. 1993;13:785-788. [PubMed] |
| 18. | Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464-4468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 666] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 19. | Farram E, Nelson DS. Mouse mast cells as anti-tumor effector cells. Cell Immunol. 1980;55:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Norrby K. Mast cells and angiogenesis. APMIS. 2002;110:355-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147:564-573. [PubMed] |
| 22. | Meininger CJ, Zetter BR. Mast cells and angiogenesis. Semin Cancer Biol. 1992;3:73-79. [PubMed] |
| 23. | Grizzi F, Franceschini B, Chiriva-Internati M, Liu Y, Hermonat PL, Dioguardi N. Mast cells and human hepatocellular carcinoma. World J Gastroenterol. 2003;9:1469-1473. [PubMed] |
| 24. | Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, Gruber BL. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J Clin Invest. 1997;99:2691-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 321] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 743] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 26. | Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, Nomura Y. Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol. 2000;7:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 219] [Article Influence: 8.8] [Reference Citation Analysis (0)] |