INTRODUCTION
Angiogenic response occurs through the release of various angiogenic factors such as vascular endothelial growth factor (VEGF). VEGF is known to be synthesized by endothelial cells, leukocytes, and megakaryocytes[1]. Angiogenesis is essential for the growth and survival of solid tumors and VEGF plays a predominant role in inducing tumor-associated angiogenesis[2,3]. Generally, VEGF is detected in most malignant epithelial tumor cells and it has been reported that the level of VEGF expression in tumor tissue is of prognostic value[4,5]. Some groups reported that both serum and plasma VEGF concentrations correlate with tumor stage[6,7]. Megakaryocytes are an important source of VEGF, which is stored in the α granules of platelets[8]. A close correlation between platelet counts and serum VEGF has been reported in cancer patients and this phenomenon is presumably due to the release of VEGF following platelet activation during the clotting process[9,10].
A VEGF-specific tyrosine kinase receptor, VEGF receptor-1 (VEGFR1/Flt-1), is found in vascular endothelial cells of tumor vessels[2,3,11]. A naturally occurring soluble form of VEGFR1 (sVEGFR1) is an alternatively spliced variant of the VEGFR1 gene and can be secreted into the circulation[5,12]. VEGFR1 has a high affinity for VEGF, even in soluble form[12]. sVEGFR1 can be derived not only from vessels but also from tumor cells[13-15]. sVEGFR1 appears to be a crucial, intrinsic, negative regulator of VEGF and it may play a key role in tumor angiogenesis[16,17].
The expression of VEGF and VEGFR1 in tissues of cholangiocarcinoma has been examined histopathologically[18]. VEGF is strongly expressed in malignant cholangiocytes, whereas the surrounding mesenchymal and endothelial cells are consistently negative for VEGF. On the other hand, VEGFR1 is expressed exclusively by endothelial cells adjacent to tumor cells and rarely expressed in quiescent endothelial cells. Endothelial cells are demonstrated as the only cellular source of the receptor. However, little is known about the clinical significance of serum levels of VEGF and sVEGFR1 in biliary diseases, especially in benign disease. The current study was therefore, initiated to evaluate the serum values of VEGF and sVEGFR1 in patients with biliary diseases.
MATERIALS AND METHODS
Sera were collected from patients with biliary disease at the Third Department of Internal Medicine and the First Department of Surgery, Kyushu University Hospital, between 2001 and 2003. Patients were divided into three groups: Group 1. Cholangitis with active inflammation (n = 42); Group 2. Biliary neoplasms including intrahepatic and extrahepatic cholangiocarcinoma (n = 44), gallbladder carcinoma (n = 7), and ampullary carcinoma (n = 8); Group 3. Vanishing bile duct disorder of autoimmune origin, primary sclerosing cholangitis (PSC: n = 12) or primary biliary cirrhosis (PBC: n = 13). For the control group (Group 4), serum samples from 31 healthy blood donors were assayed. Informed consent was obtained from each patient prior to their entering the study.
Serum concentrations of VEGF and sVEGFR1 were quantified using the high-sensitivity ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s specifications. The sensitivity of VEGF and sVEGFR1 assays was 9 pg/mL. C-reactive protein (CRP) was used as a laboratory marker of inflammation. The number of platelets and leukocytes in peripheral blood was routinely tested. Statistical analyses were performed using Spearman’s correlation analysis, Kruskal-Wallis test with Bonferroni correlation for multiple comparisons, or Wilcoxon’s signed rank test and the results were considered statistically significant if P was less than 0.05.
RESULTS
Correlation of VEGF levels with platelet counts
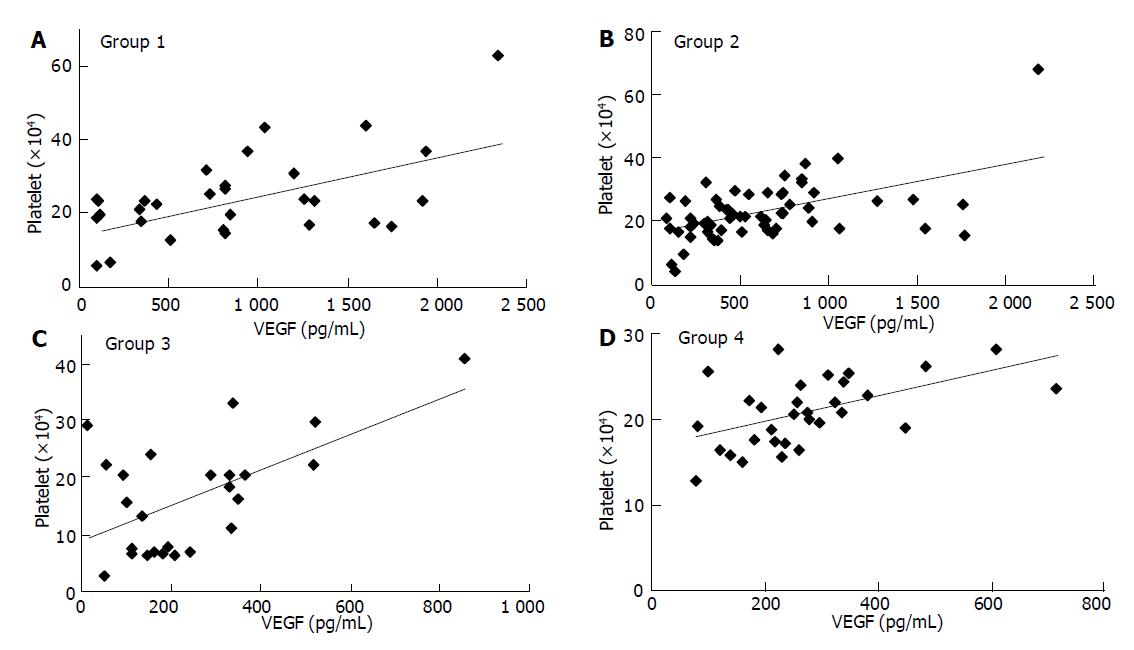
In this study, blood donors formed one of these four groups: Group 1. patients with acute cholangitis/cholecystitis; Group 2. patients with biliary carcinoma; Group 3. PBC or PSC; Group 4. healthy volunteers (see Materials and Methods). Correlations between pairs of parameters were tested by Spearman’s correlation analysis. Overall, VEGF levels showed a close relationship with platelet counts: Group 1. r = 0.449, P = 0.0029; Group 2, r = 0.439, P = 0.0005; Group 3. r = 0.393, P = 0.0520; Group 4. r = 0.537, P = 0.0018 (Figure 1). On the other hand, VEGF levels were not significantly correlated with WBC counts in healthy donors and patients with benign biliary disease: Group 1 r = 0.166; Group 3 r = 0.325; Group 4, r = 0.312. However, the correlation was significant in patients with malignancy: Group 2, r = 0.487, P<0.0001. These results indicated that platelet counts could considerably control serum VEGF levels in patients. Therefore, the concentration of VEGF per platelet (VEGF/PLT, pg/106) was calculated by dividing the serum VEGF values (pg/mL) by the platelet counts (×106/mL) in order to standardize and accurately compare the VEGF levels between patients. When VEGF levels were expressed relative to platelet counts, WBC counts did not show a significant correlation: Group 1, r = -0.049; Group 2, r = 0.288; Group 3, r = -0.076; Group 4, r = 0.205. There was no significant correlation between platelet and leukocyte counts (data not shown). The correlation was not significant between serum levels of sVEGFR1 and platelet or leukocyte counts.
Figure 1 Correlation between serum VEGF levels and platelet counts.
A: group 1; B: group 2; C: group 3; D: group 4.
Serum levels of VEGF, sVEGFR1, and VEGF/PLT
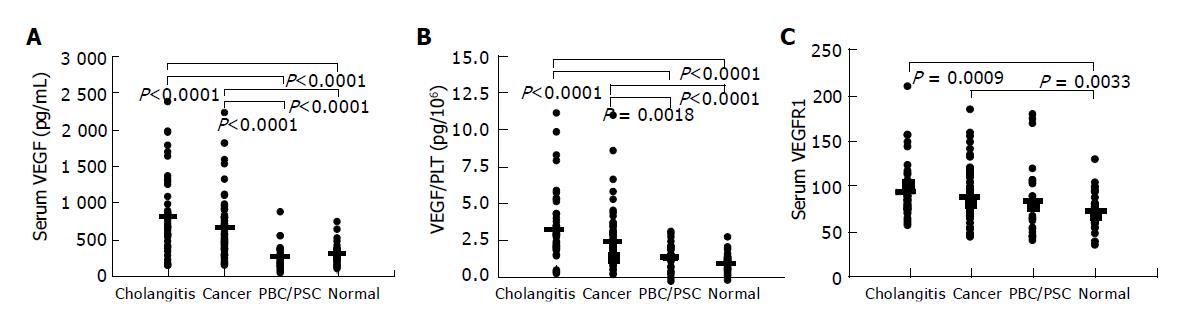
Serum levels of VEGF, sVEGFR1, and VEGF/PLT were compared among groups by Kruskal-Wallis test (Bonferroni correlation for multiple comparisons). Data for each group were presented as (median [25th. percentile, 75th. percentile]; mean value). VEGF values were significantly higher in Group 1 (648.5 [390, 1066]; 805.6 pg/mL) and Group 2 (504 [347, 775]; 633.7 pg/mL) than in Group 3 (191 [113, 333]; 246.7 pg/mL) and Group 4 (261 [185, 338]; 278.2 pg/mL) (Figure 2A). VEGF/PLT levels showed the same pattern as VEGF, and were significantly higher in Group 1 (3.22 [2.33, 4.59]; 3.78 pg/106) and Group 2 (2.51 [1.84, 3.26]; 2.86 pg/106) than in Group 3 (1.78 [1.02, 2.35]; 1.74 pg/106) and Group 4 (1.26 [0.89, 1.52]; 1.28 pg/106) (Figure 2B). sVEGFR1 levels were significantly higher in Group 1 (72 [62, 88]; 81.2 pg/mL) and Group 2 (72 [59, 90]; 77.0 pg/mL) than in Group 4 (56 [47, 74]; 60.4 pg/mL) (Figure 2C).
Figure 2 VEGF (A), VEGF/PLT (B), and VEGFR1 (C) levels in patients with biliary disease and healthy volunteers.
The mean value of each group is presented as a bold line. VEGF/PLT: the calculated parameter by dividing the serum VEGF values (pg/mL) by the platelet counts (×106/mL); Cholangitis: patients with acute cholangitis or cholecystitis; Cancer: patients with biliary neoplasms; PBC/PSC: patients with primary biliary cirrhosis or primary sclerosing cholangitis; normal: healthy volunteers.
Influence of inflammation
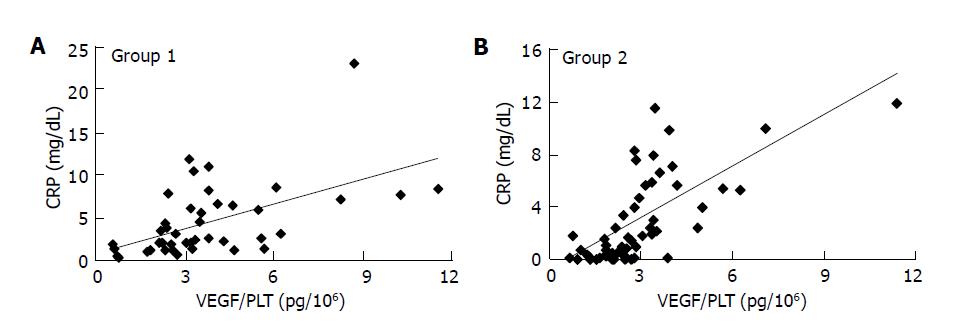
The correlation between CRP, a commonly used marker of inflammation, and VEGF/PLT or sVEGFR1 was analyzed in patients (groups 1-3) by Spearman’s correlation analysis. CRP levels were normally distributed within each group. In acute inflammatory disease (group 1), CRP and VEGF/PLT were closely correlated (r = 0.575, P<0.0001) (Figure 3A), but there was no correlation between CRP and sVEGFR1 (r = -0.067). In patients with chronic autoimmune disease, the PBC and PSC group, no significant correlation was found between CRP and VEGF/PLT (r = -0.084) or sVEGFR1 (r = 0.398). In cancer patients, significant correlations were observed between CRP and VEGF/PLT (r = 0.717, P<0.0001) (Figure 3B), and also between CRP and sVEGFR1 (r = 0.318, P = 0.018).
Figure 3 Correlation between C-reactive protein (CRP) and VEGF/PLT levels in group 1 (A) and group 2 (B).
VEGF/PLT: the calculated parameter by dividing the serum VEGF values (pg/mL) by the platelet counts (×106/mL).
Movement of serum VEGF/PLT and sVEGFR1 in cancer patients
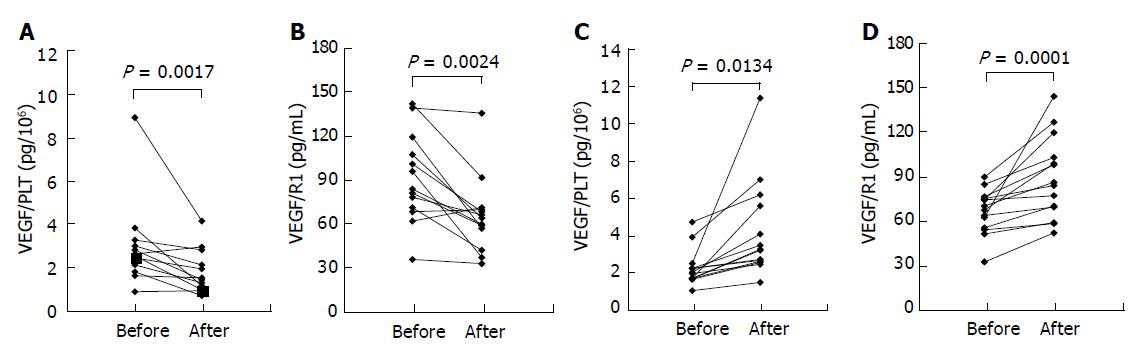
In almost all our patients, biliary carcinoma was clinically detected and diagnosed at advanced stages (stage IV or III). Therefore, patients with cancer were divided into two groups for the convenience of clinical severity: Group A, patients treated by curative surgical resection (n = 13); Group B, patients with no effective treatment (n = 14). Significant difference was tested by Wilcoxon’s signed-rank test. The levels of VEGF/PLT and sVEGFR1 were measured before and 4 wk after surgical operation in group A. In group B, the parameters were measured at intervals of 3 mo. At the time of admission to our hospital, there was no significant difference in the levels of VEGF/PLT and sVEGFR1 between Groups A and B. However, in Group A, the levels of VEGF/PLT (P = 0.0001) and sVEGFR1 (P = 0.0024) decreased significantly after surgical treatment (Figures 4A, B). By contrast, the levels of both significantly increased in Group B: VEGF/PLT, P = 0.0134; sVEGFR, P = 0.0017 (Figures 4C, D).
Figure 4 Time course of VEGF/PLT (A and C) and VEGFR1 (B and D) levels in patients with biliary cancer.
The levels were measured in each patient before and after the curative operation (A and B) or before and after the progression of cancer in size without any effective treatments (C and D). VEGF/PLT: the calculated parameter by dividing the serum VEGF values (pg/mL) by the platelet counts (×106/mL).
DISCUSSION
Recent studies have shown that serum VEGF levels are closely related to platelet counts in cancer patients[1,7,19,20]. It has been suggested that serum VEGF levels reflect platelet counts rather than VEGF secretion from tumor cells, or that platelets act as a scavenger through endocytosis and storage of VEGF secreted from the tumor[1,7,10,19-21]. We demonstrated, in this study, the relationship between serum VEGF and platelet counts is also true in biliary cancer (r = 0.439, P = 0.0005). On the other hand, some groups have not found such a correlation between VEGF and platelet counts in benign disease and healthy controls[13,14]. However, in our study, a correlative trend was obvious in acute cholangitis (r = 0.449, P = 0.0029), autoimmune biliary disease (r = 0.393, P = 0.052), and normal controls (r = 0.537, P = 0.0018). Therefore, we reasoned that expressing VEGF levels relative to platelet counts is a more meaningful way to compare the clinical significance of VEGF among diseases. Leukocytes are another known producer of VEGF. However, when WBC counts were compared with VEGF/PLT, no correlation was found in any of the groups. This finding indicates that WBC counts in peripheral blood do not significantly influence the levels of VEGF.
In our study, the levels of VEGF/PLT were higher (P = 0.0152) in acute cholangitis patients (mean value: 3.78 pg/106) than in cancer patients (mean value: 2.86 pg/106) and closely correlated with serum CRP levels (r = 0.575, P<0.0001). Angiogenesis is a necessary process for inflammation and tissue regeneration. It has been reported that inflammation and hypoxic change with expression of various tissue factors, cytokines, and chemokines stimulate VEGF synthesizing cells such as platelets, immune cells, and inflammatory cells[22-24]. Acute cholangitis is a typical intensive infectious disease. Therefore, in the patients with the disease, the correlation between inflammation activity marker, CRP, and serum VEGF levels is expressed more significantly.
Surprisingly, in biliary cancer patients, VEGF/PLT is also closely correlated with CRP (r = 0.717, P<0.0001). The phenomenon raises the question whether cholangiocarcinoma cells really produce and secrete significant amounts of VEGF, since VEGF/PLT levels may be increased by the same mechanism as in cholangitis. In patients with hypervascular massive tumors like hepatocellular carcinoma, immunohistochemical VEGF expression in cancer cells and serum VEGF are quantitatively correlated[25]. These findings support the concept that VEGF production and neovascularization are absolutely needed for these tumors to survive. On the other hand, cholangiocarcinoma is a relatively hypovascular and invasive tumor, namely, inflammation may be a more significant clinical characteristic in cholangiocarcinoma than in HCC. Therefore, platelets and inflammatory cells may play an important role in VEGF production/secretion in cholangitis as well. If this hypothesis is true, then it is not surprising that, in view of the degree of inflammation, VEGF/PLT levels were higher in the cholangitis group than in the neoplasm group.
Among the patients with autoimmune biliary disease (PBC and PSC), those at advanced stages (i.e., liver cirrhosis) showed lower platelet counts (under 1×105/μL), and therefore, the mean serum VEGF level in this group was only 234.6 pg/mL. However, no correlation was found between VEGF/PLT and CRP. These autoimmune diseases are characterized by chronic inflammatory conditions. VEGF expression is thoroughly characterized in rheumatoid arthritis (RA), a well-known systemic autoimmune disease. Serum levels of VEGF have been reported to be elevated in patients with RA and to correlate with disease activity and inflammatory markers. However, over time, the correlation with VEGF levels is only seen in RA of early to median disease duration, but not of long duration[26-28]. Generally, in patients with PBC/PSC, the clinical state is relatively stable even at advanced stages and the inflammatory events are not pronounced. Therefore, serum VEGF and VEGF/PLT levels may be in a relatively low grade and independent of CRP levels.
In our study, serum sVEGFR1 levels did not correlate with platelet and WBC counts. sVEGFR1 levels were statistically higher in groups 1 and 2 than in the normal control group. This finding can be explained by previous evidence showing that endothelial sVEGFR1 is up-regulated by its ligand, VEGF[13]. Indeed sVEGFR1 levels tend to change with VEGF/PLT level.
We have investigated whether the levels of VEGF/PLT and sVEGFR1 reflect the effect of surgical treatment in patients with biliary carcinoma. If not treated, both levels were elevated with the lapse of time. However, the levels clearly declined as a consequence of curative surgical resection. The results indicate that these serum proteins might be useful markers for gauging the clinical effect of various treatments on patients.