Published online Feb 28, 2005. doi: 10.3748/wjg.v11.i8.1141
Revised: July 28, 2004
Accepted: September 9, 2004
Published online: February 28, 2005
AIM: Silymarin is a potent antioxidant, antiinflammatory and anti-fibrogenic agent in the liver, which is mediated by alteration of hepatic Kupffer cell function, lipid peroxidation, and collagen production. Especially, in hepatic fibrogenesis, mast cells are expressed in chronic inflammatory conditions, and promote fibroblast growth and stimulate production of the extracellular matrix by hepatic stellate cells.
METHODS: We examined the inhibitory mechanism of silymarin on CCl4-induced hepatic cirrhosis in rats. At 4, 8, and 12 wk, liver tissues were examined histopathologically for fibrotic changes produced by silymarin treatment.
RESULTS: In the silymarin with CCl4-treated group, increase of hepatic stellate cells and TGF-β1 production were lower than in the CCl4-treated group at early stages. Additionally, at the late fibrogenic stage, expressions of TGF-β1 were weaker and especially not expressed in hepatocytes located in peripheral areas. Moreover, the number of mast cell in portal areas gradually increased and was dependent on the fibrogenic stage, but those of CCl4+silymarin-treated group decreased significantly.
CONCLUSION: Anti-fibrotic and antiinflammatory effects of silymarin were associated with activation of hepatic stellate cells through the expression of TGF-β1 and stabilization of mast cells. These results suggest that silymarin prevent hepatic fibrosis through suppression of inflammation and hypoxia in the hepatic fibrogenesis.
- Citation: Jeong DH, Lee GP, Jeong WI, Do SH, Yang HJ, Yuan DW, Park HY, Kim KJ, Jeong KS. Alterations of mast cells and TGF-β1 on the silymarin treatment for CCl4-induced hepatic fibrosis. World J Gastroenterol 2005; 11(8): 1141-1148
- URL: https://www.wjgnet.com/1007-9327/full/v11/i8/1141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i8.1141
Silymarin, a standardized extract of the milk thistle (Silybum marianum [L.] Gaertner) has a long tradition as an herbal remedy[1]. The flavonoid silymarin was introduced as a “hepatoprotective” agent a few years ago and is used clinically in Europe and Asia for the treatment of liver diseases[2]. The protective action of silymarin is explicable in terms of its capacity for trapping free radicals and has a stabilizing effect on the cytoplasmic membranes. In experimental animals, this flavonoid has a protective action on the liver, which is particularly vulnerable to poisoning by several hepatotoxic substances such as carbon tetrachloride (CCl4), thioacetamide, and D-galactosamine[3]. Silymarin is a potent antioxidant that inhibits lipid peroxide formation in the liver cells[4], and possesses antiinflammatory properties mediated by alteration of hepatic Kupffer cell function[5].
Hepatic fibrosis has been noted in chronic liver disease, and is characterized by increased production and deposition of collagen, glycoproteins, and proteoglycans that compose the extracellular matrix (ECM)[6]. Availability of animal models is crucial for the study of liver fibrosis and/or cirrhosis. It is well known that hepatic cirrhosis animal models for chronic liver damage induced by CCl4 in rats produce liver fibrosis and biochemical and histological patterns that resemble human liver cirrhosis[7]. Thus, the rat model of liver cirrhosis has been useful in studying the effects of hepatoprotective drugs with therapeutic potential to be used in humans[8]. In hepatic fibrogenesis, myofibroblasts (MFBs) such as hepatic stellate cells (HSCs) are the major source of increased ECM[6]. When they are exposed to soluble factors from damaged hepatocytes and from activated Kupffer cells, MFBs will lose Vitamin A and their lipid contents and undergo activation. The activated MFBs migrate and proliferate at the site of liver injury, playing a pivotal role in the formation of fibrous tissue[9]. Among the various cytokines, TGF-β1 plays an important role as a profibrogenic factor in chronic liver disease, triggering the expression of procollagen-I and tissue inhibitor of metalloproteinases-1 (TIMP-1), key effectors of fibrogenesis. TGF-β1 is also the most potent mast cell chemo-attractant so far identified and induces mast cell migration at femtomolar (fM) concentrations. Various other cell types, e.g., monocytes, neutrophils, and fibroblasts also migrate towards TGF-β1[10].
Mast cells, which are derived from hematopoietic progenitors, leave the bone marrow and migrate to areas of inflammation. A number of factors responsible for this directional migration and tissue maturation of mast cells have been identified. These include the CXC family of chemokines, stem cell factor (also known as kit-ligand, steel factor, and mast cell growth factor), and TGF-β1[11]. Thus, the activation of mast cells and the subsequent exocytosis of granules are followed by production and secretion of cytokines and other factors that lead to leukocyte infiltration and local inflammation. Mast cell hyperplasia in the liver has also been observed in a variety of experimental models of rat liver fibrosis, such as that induced by CCl4, diethylnitrosamine, radiation, porcine serum, and bile duct resection[12]. In addition, silymarin acts to stabilize hepatocyte membranes and block receptor binding of various toxins and drugs. Antioxidant activity is also hepatoprotective in vivo and in vitro studies, showing that silymarin has free radical scavenging activity and enhances superoxide dismutase action in erythrocytes and lymphocytes[13]. Silymarin also protects against glutathione depletion and increases protein synthesis by hepatocytes when there is damage to parenchymatous tissue[14].
In this study, we examined the inhibitory mechanism of silymarin on CCl4-induced hepatic cirrhosis in rats. The objective of the present study was to observe the alteration of MFBs, TGF-β1, and mast cells histopathologically on the silymarin treatment and to elucidate the correlation between these changes and the antifibrotic effect of silymarin.
Studies were performed on thirty male Wistar rats weighing 130-150 g. They were housed in a room at 22±2 °C and a 12-h light-dark cycle. Feed (PMI Nutrition International, USA) and water were supplied ad libitum.
Hepatic fibrosis was induced experimentally by intraperitoneal injection (IP) of 1.0 mL/kg body weight of 10% CCl4 (Sigma, USA) dissolved in olive oil (Sigma, USA), three times a week for 12 wk. Two groups of fifteen animals each were used. These groups are schematically shown in Table 1. The first (CCl4) group received CCl4, 1.0 mL/kg IP three times a week and 0.25% carboxymethylcellulose (CMC, Sigma, USA), 1.0 mL/kg per oral, 5 d a week for 12 wk. The second (CCl4+Sily) group received CCl4, 1.0 mg/kg IP and a daily oral dose of 50 mg/kg silymarin 5 times a week. Silymarin was given as a suspension in 0.25% CMC. Five rats of each group were sacrificed at wk 4, 8, and 12 respectively.
| Group ID | Numberof animal | Treatment | Sacrificed time |
| CCl4 | 15 | CCl4, 1.0 mg/(kg·d), IP 3 times a wk 0.25% CMC, 1 mL/(kg·d), PO 5 times a wk | 4, 8, 12 wk |
| CCl4+ Sily | 15 | CCl4, 1.0 mg/(kg·d), IP 3 times a wk Silymarin, 50 mg/(kg·d), PO 5 times a wk | 4, 8, 12 wk |
Serum was collected and assayed for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using standard enzymatic assay kits. Each assay is a colorimetric assay with detection of a highly colored end product measured at 490-520 nm using autoanalyzer - UV/V is spectrophotometer (Hitachi 736-10, Hitachi, Japan). The absorbance of each end product is proportional to the enzyme’s activity.
Hydroxyproline (HYP) was determinated colorimetrically in duplicates from 0.2 g of liver tissue using a modified method of Jamall et al[15]. Briefly, the frozen tissue was homogenized in 4 mL of 6N HCl and hydrolyzed at 110 °C for 16 h. The hydrolysate was filtered, and then 30 μL aliquot of these samples was evaporated under vacuum. The sediment was dissolved in 1.2 mL of isopropanol and incubated with 0.2 mL of 0.84% chloramines-T in acetate-citrate buffer (pH 6.0) for 10 min at room temperature. Then, 1.0 mL of Ehrlich’s reagent was added and the mixture was incubated at 60 °C for 25 min. The absorbance of the sample solution was measured at 560 nm wavelength (Hitachi 736-10, Hitachi, Japan). Next, the hydroxyproline content in 100 mg of liver was calculated from the standard curve of 4-hydroxy-L-proline (Sigma, USA) (μg/100 mg liver weight).
Liver tissues from each rat were rapidly removed, fixed in 10% neutral-buffered formalin, and processed routinely. Paraffin-embedded sections were cut into 4 μm thick sections. The sections were stained with hematoxylin and eosin (HE) and with special Azan stain, for collagen fibers. In there experiments, the degree of fibrosis in each section of liver was classified as a grade 0-4[16].
Liver sections were deparaffinized in xylene, dehydrated in graded alcohol series, and for the block of endogenous peroxidase, sections were incubated in a solution of 3% hydrogen peroxide (H2O2) in methanol for 10 min. Tissue sections were washed with PBS containing 0.03% non-fat milk and 0.01% Tween 20, and then immunostained with primary antibodies for alpha-smooth muscle actin (alpha-SMA) and TGF-β1. The antigen-antibody complex was visualized by a labeled streptavidin-biotin method using a Histostatin-PLUS Bulk Kit (Zymed Laboratories Inc., USA) and followed by diaminobenzidine (DAB) as a chromogen. After washing, slides were counter-stained with Meyer’s hematoxylin and washed with tap water. The primary antibodies used were monoclonal anti α-SMA at a dilution of 1:800 (clone 1A4, Sigma, USA) and polygonal rabbit LC[1-30] antibody to mature TGF-β1 (kindly provided by Dr. Seong-Jin Kim) at a dilution of 1:100 respectively. Non-immunized goat sera, which were used instead of the primary antibody, served as the negative control.
Toluidine blue staining for mast cells was performed by immersion of liver sections in 0.1% toluidine blue (Sigma, USA) for 1 min at room temperature. The number of mast cells was quantified in 25 randomly selected, non-overlapping fields and expressed as the number of mast cells/mm2.
All results were expressed as mean and standard deviation (SD). Statistical analysis of the data was done using InStat program (GraphPad Software Inc.). A Mann-Whitney U-test was conducted and the data that were considered to be significantly different were reported at probability levels of P<0.05 or P<0.005, as indicated.
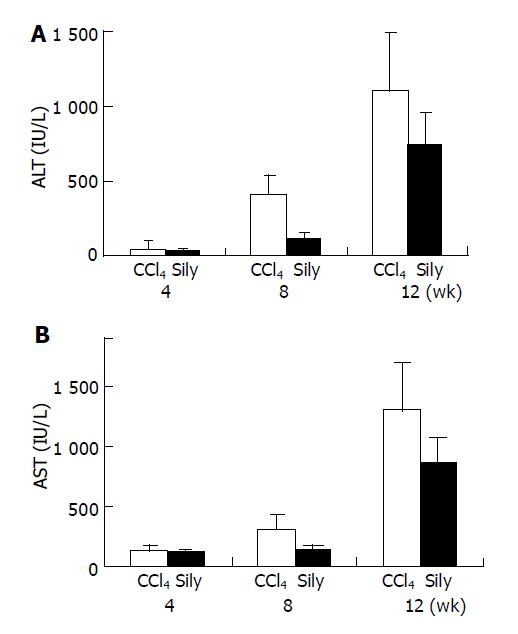
Serum ALT and AST levels of all CCl4-tested animals increased in a time-dependent fashion during the whole of experimental period (Figure 1). These changes indicated that liver damage and inflammation were induced successfully by injection of CCl4 and were similar to previous results. However, in the CCl4+silymarin treatment group, increases of serum ALT and AST levels were lower than those of the CCl4, control group. The reduced ALT and AST activities of the silymarin-treated group were 30%, 72% and 33% compared to increases of 8%, 55% and 34% in the CCl4-treated groups, at 4, 8, and 12 wk.
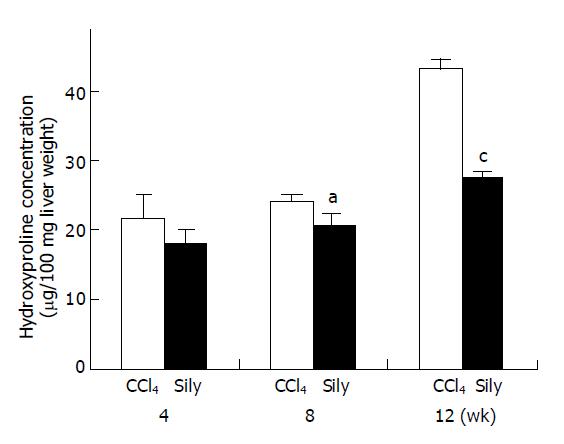
Collagen contents of liver tissue were quantified by determination of the hydroxyproline content (Figure 2). In the CCl4-treated group, hydroxyproline contents at 4, 8, and 12 wk increased 21.81±3.37, 24.31±0.91, and 43.26±1.41 μg/100 mg liver weight respectively. However, in the CCl4+silymarin-treated group, the HYP contents at 4, 8, and 12 wk were 18.05±1.89, 20.5±1.92, and 27.5±0.69 μg/100 mg liver weight respectively. At 4, 8, and 12 wk, these HYP contents of CCl4+silymarin-treated group were reduced by 17%, 16%, and 36% respectively compared to those of control group. Reduced HYP contents of 8 and 12 wk in the CCl4+silymarin-treated group were statistically significant, P<0.05 and <0.005 respectively.
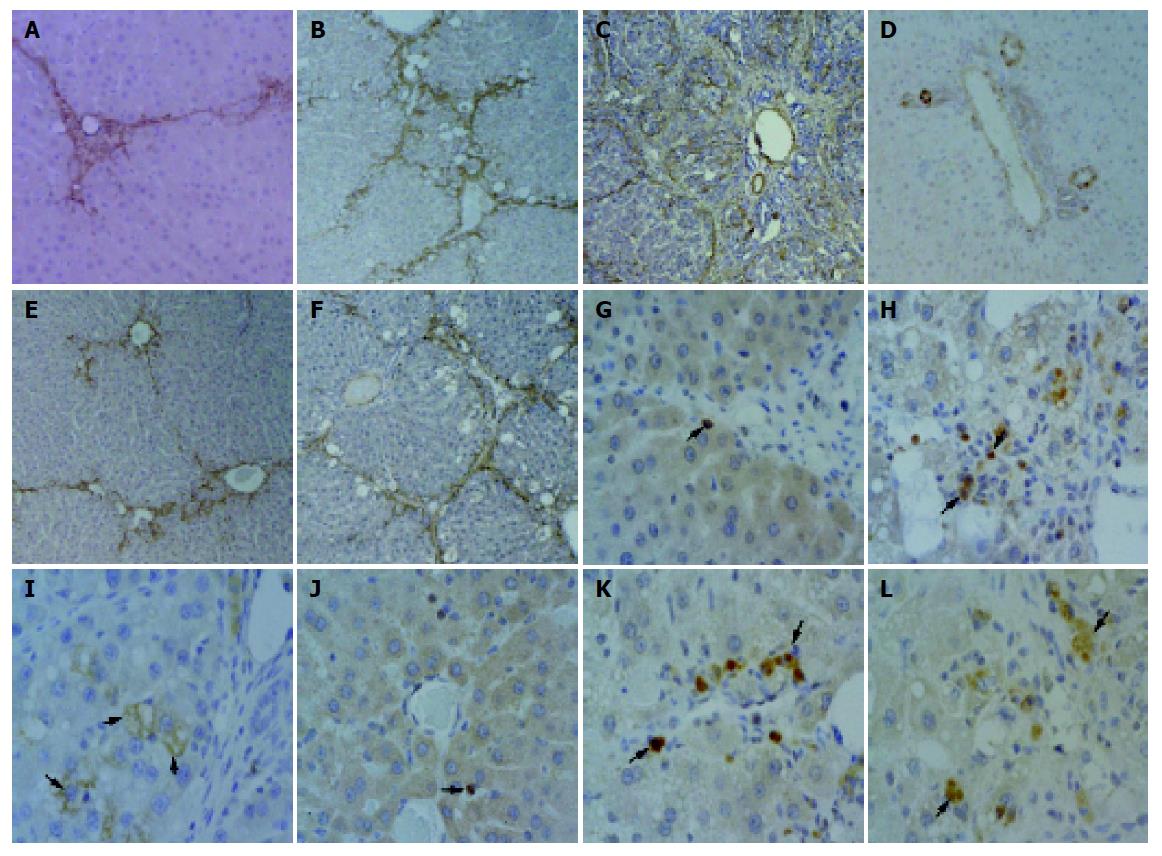
During the process of cirrhosis, the grade of hepatic fibrosis changed from grade 0 to grade 4 (Table 2). At 4 wk, centrilobular necrosis and moderate fatty change of liver were found in the CCl4-only-treated group. In this group, there was collagen accumulation around the blood vessels (Figure 3A, G). At 8 wk, fatty change was rarely observed, but collagen fibers were more abundant in the centrilobular area and neighboring central veins were bridged by fibrous septa. Then, pseudolobuli were formed by thin fibrous septa (Figure 3B, H). At 12 wk, pseudolobuli were formed and macrovesicular lipid droplets were detected. The collagenous septa were much thicker, and pseudolobuli were subdivided into smaller lobuli (Figure 3C, I).
| Group | 4 wk | 8wk | 12 wk | |||
| Grade1 | Lesion | Grade | Lesion | Grade | Lesion | |
| CCl4 | 2 | Mild fibrosis | 3 | Severe fibrosis | 4 | Cirrhosis |
| CCl4+Sily | 0-1 | Mild fatty change | 1-2 | Mild fatty change and fibrosis | 2-3 | Severe fatty change and fibrosis |
In the silymarin with CCl4 injection group, at 4 wk, the presence of connective tissue was almost normal around the central veins (Figure 3D, J). At wk 8, moderate to severe fatty change was detected around the periportal area and central vein. A slight accumulation and spread of collagen fibers around central veins was observed too (Figure 3E, K). At wk 12, large lipid accumulation was detected more than that of the CCl4-treated group and pseudolobuli were formed by thin collagenous septa (Figure 3F, L). The presence of collagenous fibers, as demonstrated by Azan stain, was confirmed too.
Normal expression of myofibroblasts (MFBs) (e.g., hepatic stellate cells, HSCs, etc.) was identified by α-SMA-positive staining and was limited to the central veins and portal triad. As liver damage, progressed α-SMA-positive cells markedly increased around blood vessels and fibrotic tissue in the CCl4-only-treated group at wk 4, 8, and 12 (Figure 4A-C). In the silymarin with CCl4-treated group, at 4 wk, α-SMA-positive cells were slightly detected around central and portal veins, the same as in the control group (Figure 4D), and then at wk 8 increasing α-SMA-positive MFBs exhibited the same pattern of collagen fiber spread from the central veins (Figure 4E). However, the numbers of α-SMA-positive cells in the silymarin with CCl4-treated group was lower than in the CCl4-only-treated group at wk 8 (Figure 4B, E). At wk 12, the CCl4-treated group developed into a cirrhotic stage, and α-SMA-positive MFBs were observed mild along to the thick collagenous septa (Figure 4C), compared to 8 wk. This indicated expression of the collagen matrix in the hepatocytes against activation of myofibroblasts, which disappeared at the stage of cirrhosis. However, at 12 wk, MFBs of the CCl4+silymarin-treated group showed positive expression of α-SMA identical to the pattern in fibrosis (Figure 4F).
During hepatic fibrogenesis in CCl4-treated group, expression of TGF-β1 was detected in bile ductular epithelial cells and macrophages at 4 and 8 wk (Figure 4G, H). As the fibrotic grade increased, the positive reaction of TGF-β1 was predominantly expressed in the macrophages and MFBs. Significantly, at 12 wk several hepatocytes showed TGF-β1-positive immunoreaction. These hepatocytes were located peripherally within the pseudolobules (Figure 4I). However, in the CCl4+silymarin-treated group, the expression of TGF-β1 was weaker than in the CCl4-treated group (Figure 4J-L).
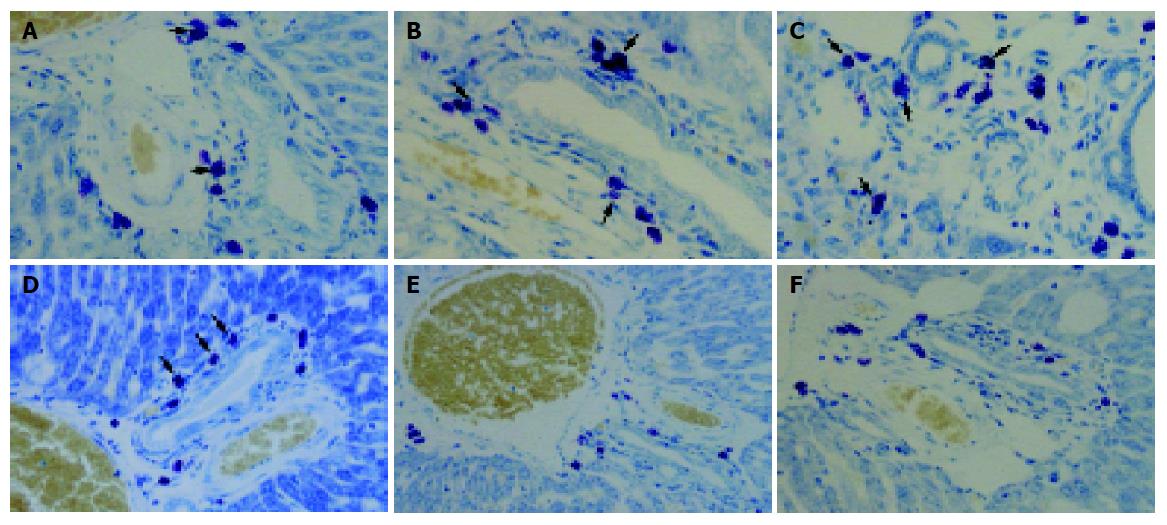
In toluidine blue stained liver sections from the CCl4-treated group mast cells were oval in shape with metachromatic granules in portal areas (Figure 5). At 4, 8, and 12 wk, the number of mast cells of the CCl4-treated group increased gradually: 12.43±2.38, 18.17±2.12 and 21.8±4.92 cells/mm2 respectively. However, in the CCl4+silymarin-treated group, the number of mast cell were 10±3.33, 12.42±2.35, and 12.17±3.07 cells/mm2 respectively (Figure 6). These numbers for the CCl4+silymarin-treated group decreased significantly by 19%, 32%, and 44% compared to those of CCl4-treated group, indicating a silymarin-mediated protective mechanism.
Silymarin is well known to be a protective agent against various hepatotoxins, such as acetaminophen, alcohol, carbon tetrachloride, tetrachloromethane, and toluene[17]. Pretreating rats and mice with silymarin before exposure to these chemical hepatotoxins significantly reduced lipid peroxidation and hepatotoxicity[3]. Additionally, the pharmacological effects of silymarin include regulation of cell membrane permeability, leukotriene inhibition, reactive oxygen species scavenging, and suppression of NF-kappaB DNA binding activity[18].
In animal studies, silymarin is as effective as colchicine in reversing hepatic fibrosis due to CCl4-induced damage[1]. A CCl4-induced hepatic cirrhosis rat model has been useful in studying the effects of hepatoprotective drugs with therapeutic potential to be used in humans[8]. In the current study, hepatic fibrosis/cirrhosis was successfully induced by CCl4 injection in rats and flavorous results were obtained similar to previous study. Total hepatic collagen contents determined by hydroxyproline content increased gradually during experimental period and histopathological findings of fibrosis/cirrhosis were observed in H&E and the Azan-stained section. In the present study, the hepatoprotective effect of silymarin was determined in CCl4-induced liver cirrhosis of rats. In the CCl4 with silymarin-treated group, total hepatic collagen contents were significantly lower than in the CCl4-treated group especially at 8 and 12 wk. Additionally histopathological findings of fibrosis/cirrhosis were revealed a significant reduction in the CCl4-treated group. Similar experiments by Favari et al reported the reduction of lipid peroxidation, Na+, K+, and Ca2+-ATPase levels and increases of collagen content[3,19].
In hepatic fibrogenesis, myofibroblasts (MFBs) are the major source of increased ECM. The activated MFBs migrate and proliferate at the site of liver injury and play a pivotal role in the formation of fibrous tissue. Therefore, activated MFBs are considered the major cellular target to prevent the progression of liver fibrosis during the new drug development[9]. In addition, transforming growth factor beta (TGF-β) is a potent fibrogenic cytokine produced by Kupffer cells and HSCs. There is a prolonged increase of TGF-β1 expression during hepatic fibrosis in CCl4- and diethylnitrosamine-induced models[20] and in patients with cirrhosis induced by alcohol or viral hepatitis[21].
In the current study, as liver damage progressed in the CCl4-only-treated group, α-SMA-positive cells markedly increased around fibrous septa. The number of these cells increased from wk 4 to 8, but slightly decreased along the thick collagenous septa at 12 wk, developed into the cirrhotic stage. Characteristically, in immunohistochemical analysis for TGF-β1, positive reactions were mainly expressed by HSC and macrophages around the portal region, at early (4 wk) and middle (8 wk) stages of the fibrotic processes, but they were predominantly observed in hepatocytes located in pseudolobules peripherally, at the cirrhosis-occurred stage (12 wk). The mechanism of TGF-β1 expression in hepatocytes has been studied by several researchers, but is still unclear. Furthermore, these alterations of TGF-β1 expression were already reported by our laboratory and we suggested that hypoxia might be associated with fibrogenesis in the liver[22]. However, in the silymarin+CCl4-treated group of our study, there was increase of α-SMA-positive cells such as MFBs, lower than that of CCl4-only-treated group, at 4, 8, and 12 wk. Additionally, expressions of TGF-β1 were weaker than those of CCl4-treated group, during all experimental periods, especially at 12 wk not expressed in hepatocyte located in peripheral areas of pseudolobules. Thus, based on the results of the current study, it is concluded that silymarin has protective effect of proliferation and TGF-β1 production in MFBs.
Fuchs et al[23] reported on the basis of in vitro studies that the potential antifibrotic properties of silymarin might be the inhibition of hepatic stellate cell proliferation and transformation. Jia et al[24] observed that silymarin suppresses expression of profibrogenic procollagen alpha1 (I) and TIMP-1 most likely via down-regulation of TGF-β1 mRNA in rats with biliary fibrosis. However, in vivo studies of the anti-fibrotic activities of silymarin have not yet elucidated histopathologically the preventive mechanism of activation or proliferation of MFBs by silymarin during the CCl4-induced hepatic fibrogenesis. These results suggest that alterations of the numbers of MFBs and TGF-β1 expression in the liver may be involved in the hepatoprotective effects of silymarin observed in other studies. Another study of silymarin explained the antifibrotic action through the effects on TGF-β1 expression[24]. Silymarin has been noted to regenerate cells and enhance RNA synthesis in the rat liver[25].
In liver fibrosis and/or cirrhosis, several studies have reported a relationship between mast cell density, hepatocellular damage, mRNA encoding TGF-β1, hepatic stellate cell activation, and collagen levels[26-28]. Mast cells have been implicated in chronic inflammatory conditions resulting in fibrosis, such as Crohn’s disease, and have been identified in human liver. The number of mast cells are reported to increase in chronic liver diseases associated with fibrosis[12]. Armbrust et al[29] demonstrated that in the late stage of liver fibrogenesis, mast cells may be involved by displaying protease inhibitory activity in the fibrotic septa. In our previous study, the chronic injection of CCl4 induced rat liver cirrhosis concomitant with a marked increase of mast cells[30]. In this study, the number of mast cells in portal areas gradually increased in the fibrogenic stage, but the number of mast cells in the CCl4+silymarin-treated group decreased significantly compared to those of CCl4-treated group. In the study of Fantozzi et al, there was inhibition of neutrophil-mediated histamine release dose-dependently. These results further stress the concept of a neutrophil-mast cell interaction, which may be involved in inflammatory processes[31]. Moreover, mast cells secrete various mediators, which promote fibroblast growth, stimulate production of the extracellular matrix by fibroblasts of hepatic stellate cells, and produce components of the extramedullary matrix themselves[32,33]. However, it is unclear whether they play a central role in its development.
The anti-inflammatory effects of silymarin are also based on multiple activities including mast cell stabilization, inhibition of neutrophil migration, Kupffer cell inhibition, inhibition of leukotrienes, and prostaglandin formation. However, results of earlier studies have not histopathologically shown expression of mast cells in fibrotic liver tissue after silymarin treatment. Thus, silymarin has been histopathologically shown to have significant antiinflammatory effect on hepatic tissue, including mast cell stabilization. In addition, it is likely that the hepatoprotective effect of silymarin is related to prevention of hypoxia in hepatic fibrogenesis.
In conclusion, the anti-fibrotic and antiinflammatory effects of silymarin were histopathologically observed in the hepatic fibrogenesis of chronic liver damage induced by CCl4 treatment. Furthermore, these effects were associated with activation of MFBs, expression of TGF-β1, and stabilization of mast cells. These results suggest that silymarin prevents hepatic fibrosis through the suppression of inflammation and hypoxia in CCl4-induced rat liver cirrhosis.
| 1. | Valenzuela A, Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res. 1994;27:105-112. [PubMed] |
| 2. | Down WH, Chasseaud LF, Grundy RK. Effect of silybin on the hepaticmicrosomal drug metabolizing enzyme system in the rat. Arzneim-Forsch. Drug Res. 1974;24:1986-1988. |
| 3. | Mourelle M, Muriel P, Favari L, Franco T. Prevention of CCl4-induced liver cirrhosis by silymarin. Fundam Clin Pharmacol. 1989;3:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Akiyoshi H, Terada T. Mast cell, myofibroblast and nerve terminal complexes in carbon tetrachloride-induced cirrhotic rat livers. J Hepatol. 1998;29:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983;3:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 198] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Paquet KJ, Kamphausen U. The carbon-tetrachloride-hepatotoxicity as a model of liver damage. First report: Long-time biochemical changes. Acta Hepatogastroenterol (Stuttg). 1975;22:84-88. [PubMed] |
| 9. | Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Olsson N, Piek E, ten Dijke P, Nilsson G. Human mast cell migration in response to members of the transforming growth factor-beta family. J Leukoc Biol. 2000;67:350-356. [PubMed] |
| 11. | Jones SE, Kelly DJ, Cox AJ, Zhang Y, Gow RM, Gilbert RE. Mast cell infiltration and chemokine expression in progressive renal disease. Kidney Int. 2003;64:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Sugihara A, Tsujimura T, Fujita Y, Nakata Y, Terada N. Evaluation of role of mast cells in the development of liver fibrosis using mast cell-deficient rats and mice. J Hepatol. 1999;30:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Altorjay I, Dalmi L, Sári B, Imre S, Balla G. The effect of silibinin (Legalon) on the the free radical scavenger mechanisms of human erythrocytes in vitro. Acta Physiol Hung. 1992;80:375-380. [PubMed] |
| 14. | Galisteo M, Rissel M, Sergent O, Chevanne M, Cillard J, Guillouzo A, Lagadic-Gossmann D. Hepatotoxicity of tacrine: occurrence of membrane fluidity alterations without involvement of lipid peroxidation. J Pharmacol Exp Ther. 2000;294:160-167. [PubMed] |
| 15. | Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 421] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Fujiwara K, Ogata I, Ohta Y, Hayashi S, Mishiro S, Takatsuki K, Sato Y, Yamada S, Hirata K, Oka H. Decreased collagen accumulation by a prolyl hydroxylase inhibitor in pig serum-induced fibrotic rat liver. Hepatology. 1988;8:804-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 424] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 18. | Schümann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. Silibinin protects mice from T cell-dependent liver injury. J Hepatol. 2003;39:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Favari L, Pérez-Alvarez V. Comparative effects of colchicine and silymarin on CCl4-chronic liver damage in rats. Arch Med Res. 1997;28:11-17. [PubMed] |
| 20. | Nakatsukasa H, Nagy P, Evarts RP, Hsia CC, Marsden E, Thorgeirsson SS. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990;85:1833-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Annoni G, Weiner FR, Zern MA. Increased transforming growth factor-beta 1 gene expression in human liver disease. J Hepatol. 1992;14:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Jeong WI, Do SH, Yun HS, Song BJ, Kim SJ, Kwak WJ, Yoo SE, Park HY, Jeong KS. Hypoxia potentiates transforming growth factor-beta expression of hepatocyte during the cirrhotic condition in rat liver. Liver Int. 2004;24:658-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Fuchs EC, Weyhenmeyer R, Weiner OH. Effects of silibinin and of a synthetic analogue on isolated rat hepatic stellate cells and myofibroblasts. Arzneimittelforschung. 1997;47:1383-1387. [PubMed] |
| 24. | Jia JD, Bauer M, Cho JJ, Ruehl M, Milani S, Boigk G, Riecken EO, Schuppan D. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen alpha1(I) and TIMP-1. J Hepatol. 2001;35:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Sonnenbichler J, Zetl I. Mechanism of action of silibinin. V. Effect of silibinin on the synthesis of ribosomal RNA, mRNA and tRNA in rat liver in vivo. Hoppe Seylers Z Physiol Chem. 1984;365:555-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Grizzi F, Franceschini B, Gagliano N, Moscheni C, Annoni G, Vergani C, Hermonat PL, Chiriva-Internati M, Dioguardi N. Mast cell density, hepatic stellate cell activation and TGF-beta1 transcripts in the aging Sprague-Dawley rat during early acute liver injury. Toxicol Pathol. 2003;31:173-178. [PubMed] |
| 27. | Li CY, Baek JY. Mastocytosis and fibrosis: role of cytokines. Int Arch Allergy Immunol. 2002;127:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Zheng M, Ruan Y, Wu Z. Correlation study of TGF beta expression in diethylnitrosamine-induced rat liver cancer and mast cells in its vicinity. Zhonghua ZhongLiu ZaZhi. 2000;22:463-465. [PubMed] |
| 29. | Armbrust T, Batusic D, Ringe B, Ramadori G. Mast cells distribution in human liver disease and experimental rat liver fibrosis. Indications for mast cell participation in development of liver fibrosis. J Hepatol. 1997;26:1042-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Jeong WI, Lee CS, Park SJ, Chung JY, Jeong KS. Kinetics of macrophages, myofibroblasts and mast cells in carbon tetrachloride-induced rat liver cirrhosis. Anticancer Res. 2002;22:869-877. [PubMed] |
| 31. | Fantozzi R, Brunelleschi S, Rubino A, Tarli S, Masini E, Mannaioni PF. FMLP-activated neutrophils evoke histamine release from mast cells. Agents Actions. 1986;18:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Hatamochi A, Fujiwara K, Ueki H. Effects of histamine on collagen synthesis by cultured fibroblasts derived from guinea pig skin. Arch Dermatol Res. 1985;277:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Weber S, Krüger-Krasagakes S, Grabbe J, Zuberbier T, Czarnetzki BM. Mast cells. Int J Dermatol. 1995;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |