Published online Feb 21, 2005. doi: 10.3748/wjg.v11.i7.931
Revised: July 26, 2004
Accepted: October 20, 2004
Published online: February 21, 2005
Infection with human papillomaviruses is strongly associated with the development of multiple cancers including esophageal squamous cell carcinoma. The HPV E6 gene is essential for the oncogenic potential of HPV. The regulation of apoptosis by oncogene has been related to carcinogenesis closely; therefore, the modulation of E6 on cellular apoptosis has become a hot research topic recently. Inactivation of the pro-apoptotic tumor suppressor p53 by E6 is an important mechanism by which E6 promotes cell growth; it is expected that inactivation of p53 by E6 should lead to a reduction in cellular apoptosis, numerous studies showed that E6 could in fact sensitize cells to apoptosis. The molecular basis for apoptosis modulation by E6 is poorly understood. In this article, we will present an overview of observations and current understanding of molecular basis for E6-induced apoptosis.
- Citation: Li TT, Zhao LN, Liu ZG, Han Y, Fan DM. Regulation of apoptosis by the papillomavirus E6 oncogene. World J Gastroenterol 2005; 11(7): 931-937
- URL: https://www.wjgnet.com/1007-9327/full/v11/i7/931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i7.931
Papillomaviruses are small DNA viruses that infect various epithelial tissues. Papillomaviruses replicate in the stratified layers of skin and mucosa, and usually give rise to benign lesions such as warts or papillomas. Human papillomaviruses (HPVs) can be classified as either high-risk or low-risk type on the basis of their clinical associations. The high-risk HPV types, of which type 16 (HPV-16) is the most prevalent type, are commonly associated with lesions that can progress to high-grade intraepithelial neoplasia and ultimately to carcinoma, while the low-risk HPV types, such as HPV-6 and -11, are found associated primarily with benign lesions, which rarely progress to cancer[1]. A subgroup of low risk HPV types, including HPV-5 and -8, are frequently detected in skin cancers that develop from multiple flat warts when combined with certain physical and chemical carcinogens[2].
Squamous cell cancer of Esophagus is the pathological type that is most closely associated with HPV infection in gastrointestinal malignancies. Syrjanen group found condyloma-like lesions in the specimens of esophageal cancer in 1982, which links HPV infection to esophageal cancer for the first time[3]. This finding was soon substantiated by the demonstration of HPV structural proteins in these lesions using immunohistochemistry[4]. Numerous reports have been published since then. However, there is a wide variation on HPV infection rates among different studies, ranging from 0% to 88%, which make it still hard to consolidate the role of HPV (Table 1). This variation seems to be influenced by methodology of detection, pathological grading, geographic distribution and genetic sensitivity to HPV infection. Even though different opinions do exist, a large portion of them strongly suggest a causal role for HPV in esophageal carcinogenesis, or at least consider HPV as a possible contributor in those HPV prevalent areas such as China and South Africa.
| Area or Country | HPV positive | Dectection method | Reference | |
| % | n | |||
| Germany | 0 | 0/23 | PCR | [90] |
| Japan | 0 | 0/4 | IHC | [91] |
| Italy | 4.4 | 2/45 | PCR | [92] |
| United States | 4.5 | 1/22 | PCR | [93] |
| Belgium | 4.8 | 1/21 | PCR | [94] |
| Linxian, China | 6.7 | 2/32 | PCR | [95] |
| Japan | 16 | 12/75 | PCR | [96] |
| Northern China | 16.8 | 17/101 | PCR | [97] |
| Northern China | 16.9 | 118/700 | ISH | [6] |
| Cixian, China | 20.3 | 26/128 | PCR | [98] |
| Hungary | 39 | 32/82 | PCR | [99] |
| Japan | 42 | 20/48 | PCR | [100] |
| South Africa | 46 | 23/50 | PCR | [101] |
| Italy | 47 | 8/17 | PCR | [102] |
| Anyang, China | 63.3 | 19/30 | PCR | [103] |
| Shanxi and Anyang, China | 64 | 31/48 | PCR | [7] |
| Guangdong, China | 65.5 | 96/176 | PCR | [104] |
| Beijing, China | 70 | 28/40 | ISH | [105] |
| Northern India | 74 | 20/27 | PCR | [106] |
| Beijing, China | 83.3 | 15/18 | PCR | [107] |
| Mexico | 88 | 20/23 | PCR | [14] |
The incidence of esophageal cancer in Anyang area is one of the highest in China, with a mortality rate of 132×105, significantly higher than the one of 52×105 in neighboring area. A 132-case survey in this area showed that the infection rate of HPV-16 is much higher than neighboring area, 1.9 fold by PCR (72% vs 37%) and 2.2 fold by immunohistochemisty (49% vs 22%), and the infection of HPV is closely related with the degree of dysplasia[5-26]. Compared to normal adjacent tissues, samples from esophageal carcinoma showed significantly higher infection rate for HPV[13,20].The most frequently detected types of HPV in esophageal cancer are HPV-16 and -18[27].
Some indirect or direct evidences have been shown recently to further substantiate the causal role of HPV. When the genomes of HPV-16 and -18 without E1 and E2 were transfected transiently into esophageal cancer cell, these viral genomes replicated in the absence of E1 and E2, which suggest specific host nuclear factors in esophageal squamous epithelial cells may support HPV replication[28]. Other researchers have reported that E6 gene can actually associate with the nuclear matrix of esophageal carcinoma cell. Evidence from animal studies showed that persistent papillomatosis and carcinomas in cattle can be experimentally reproduced with bovine papillomavirus 4 (BPV 4) infections in these animals. Up to 96% of the cancer-bearing animals have concomitant papillomas, and the progression from benign papilloma to carcinomas could be clearly identified[29]. Recently an immortal esophageal cell line was established by transferring HPV 18E6E7 into fetal esophageal epithelium; this cell line showed gradual change from preimmortal, immortal, precancerous to malignantly transformed stages upon prolonged cultivation without any co-carcinogens, which provided valuable direct proof on the role of HPV in carcinogenesis process of esophageal carcinoma[30,31].
The major role of HPV might be in the early stage of carcinogenesis since it has been shown in several studies that compared to esophagitis, precancerous leisions showed more HPV infection (96% vs 26%), while in advanced esophageal cancer specimens, the positive rate leveled off a little (88%)[25]. The hypothesis might be that HPV play its role in near-normal differentiating cells; this differentiating status is needed for HPV to replicate and when these cells acquire malignant phenotype changes step by step, the differentiation process is reversed. At this stage, HPV will have to face hostile environments to replicate. This may also explain the wide variation on positive rates when detecting HPV in esophageal cancer specimens since the pathological grading may vary greatly. In this aspect, the relation of HPV with esophageal cancer is somehow like the one of HBV with hepatocellular carcinoma. In benign tissue of infected liver such as cirrhosis, HBeAg and HBcAg were easily detected, but after malignant changes happened following virus infection, it became much harder to detect[32].
Multiple factors besides HPV are considered in carcinogenesis of esophageal cancer, such as some chemicals (nitrosamines, mycotoxins, cigarette smoke, excessive alcohol intake), nutritional deficiencies and physical factors (hot food), thus making it very hard to clearly characterize the significance of HPV in esophageal cancer. More insights will be needed to fully demonstrate the mechanisms involved. Before that it might be hard to draw a final conclusion on the causal role of HPV in esophageal carcinogenesis.
The transforming properties of high-risk HPVs primarily reside in two genes, E6 and E7, which are consistently expressed in HPV-positive cervical cancers and cancer-derived cell lines[33]. The sustained expression of E6 and E7 is essential to maintain the transformed state of HPV-positive cells[34]. Independent of E7, E6 exhibits important biological activities. The modulation of E6 in apoptosis will be the focus of this review. However, due to the technical difficulty to establish a normal esophageal keratinocyte cell line, most studies were carried out in keratinocytes from foreskin or skin, or even in unrelated cell types.
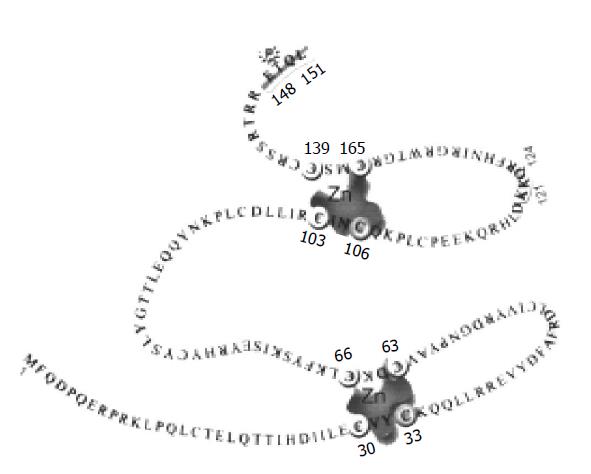
The papillomavirus E6s are relatively small proteins. For example, HPV-16 E6 protein is a small protein of 151 amino acids (Figure 1). E6 proteins from different HPV types or among the animal and human papillomaviruses show moderate amino acid homology. The common feature of most E6 proteins is the presence of four putative Cys-X-X-Cys motifs that are capable of binding zinc[35-37]. The importance of Cys-X-X-Cys motifs for E6 proteins has been implicated in functions such as transcriptional activation, transformation, immortalization, and association with cellular proteins[37-42]. There is a PDZ-binding motif in high-risk HPV’s E6 that is important for association with PDZ containing proteins[43,44]. A phosphorylation site for protein kinase A on E6 has also been identified[45].
Localization of E6 has been controversial and complex, partly due to its very low level in the cells. Nevertheless, E6 proteins have been localized to the nuclear, cytoplasmic, and non-nuclear membrane (including Golgi membrane) fractions in a variety of cells[46,47]. A recent study showed that HPV-18 E6 localization is an actively controlled process[47]. Nuclear entry of HPV-16 E6 was shown to occur via several pathways[48]. Some recent studies also revealed differences in cellular localization between E6 proteins from high-risk and low-risk HPVs[47].
Non-specific double-stranded DNA-binding by E6 has been observed in vitro[37,49]. Sequence-specifically binding to the HPV long control region has also been described for HPV-16 E6[50]. Recently, specific recognition of Holiday junctions by E6 from high-risk HPVs was demonstrated[51,52].
The oncogenic activities of E6 have been demonstrated in multiple biological assays. These include immortalization of primary human epithelial cells, transformation of established mouse fibroblasts, transcriptional activation, resistance to terminal differentiation of human keratinocytes, modulation of apoptosis, and tumorigenesis in animals[53]. Some recent studies showed that E6 played an essential role in HPV life cycle[54]. Although E6, along with E7, efficiently immortalizes primary human epithelial cells, is not sufficient in induction of human cell transformation; additional alterations are required for the cells to be fully transformed[55].
Association of E6 with p53 is mediated by the ubiquitin ligase E6AP that leads to the degradation of p53 by the ubiquitination pathway[56,57]. One of the most important p53- induced gene product is the universal cyclin-dependent kinase (CDK) inhibitor p21Waf1/Cip1[58]. Notably, posttranscriptional down regulation of p21 by E6 has also been reported in several normal cell types[59]. Consistent with these observations, differential expression of p53 and p21 in cervical squamous intraepithelial lesions infected with HPV has also been observed[60]. E6 also has functions independent of inactivating p53 and has been shown to interact with multiple additional cellular proteins[53]. These include the pro-apoptotic protein Bak, tumor necrosis factor receptor 1, and the DNA repair protein MGMT and XRCC1[61,62].
Apoptosis is a genetically programed process of cellular destruction that is indispensable for the normal development and homeostasis of multi-cellular organisms[63]. Apoptosis is characterized by plasma membrane blebbing, condensation, and fragmentation of cells and nuclei, degradation of chromosomal DNA into nucleosomal units[64]. Apoptosis serves to eliminate cells that are no longer required or potentially dangerous, such as radiation-damaged, aberrantly growing due to oncogene activation, and virally infected cells. Regulation of apoptosis is very important in terms of pathogenesis of diseases. Inappropriate occurrence of apoptosis results in neurodegenerative diseases and AIDS, while the failure of appropriate apoptosis contributes to autoimmune diseases and cancer. Many viral proteins have been found to modulate apoptosis[65]. Both pro- and anti-apoptotic activities for papillomavirus E6 have been described. While the anti-apoptotic function of E6 can be attributed in part to its ability to degrade p53, little is known regarding how E6 sensitizes cells to apoptosis.
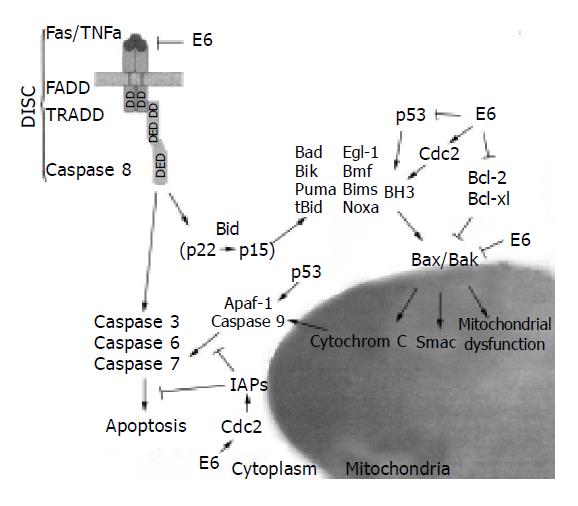
The apoptotic signal may originate endogenously, for example, from DNA damage, uncoordinated induction of cell cycle, or disruption of the cellular metabolism. This pathway involves the mitochondria and more specifically cytochrome c, the protein localized in the inner mitochondrial membrane and the inter-membrane space[66]. During apoptosis, cytochrome c is released in the cytosol and together with Apaf-1, activates procaspase 9. Activated caspase 9 then cleaves and activates the executioner caspase 3, an event that leads to the cleavage of other death substrates, cellular and nuclear morphological changes, and ultimately to cell death[67]. Apoptotic signals can also be triggered externally once the suitable surface death receptors are ligated. For example, the Fas (CD95/APO-1) receptor transduces apoptotic signals upon cross-linking with the Fas ligand (FasL). FasL binding triggers trimerization of the Fas receptor and recruitment on the cytoplasmic death domain DD of death-inducing signaling complex (DISC), which includes the adaptor FADD and pro-caspase 8 as crucial physiological death effectors. Coupling of pro-caspase 8 to Fas results in proteolytic activation of caspase 8. Two pathways have been shown for the signal transduction downstream of caspase 8, which are used in different cell types (types I and II)[68]. In type I cells, caspase 8 directly activates procaspase 3; in type II cells, caspase 8 cleaves Bid, a proapoptotic member of the Bcl-2 family[69,70]. The cleaved Bid translocates to the mitochondria and stimulates the release of cytochrome c (Figure 2).
The pathways of cell proliferation and apoptosis are tightly coupled. Inappropriate proliferation of somatic cells may trigger apoptosis. Activation of p53 by DNA damage induces either cell cycle arrest or apoptosis[71]. The cytostatic effect of p53 is largely mediated by transcriptional activation of p21, whereas the apoptotic effect is mediated by transcriptional activation of pro-apoptotic genes including BAX and PUMA[72,73]. Compared to many normal tissues, cancer cells are highly sensitized to apoptotic signals, and survive only because they have acquired lesions such as loss of p53 that prevent or impede cell death[71]. Much effort has gone into determining the effects of p53 inactivation on the response of cancer cells to the therapeutic agents. The results have been conflicting, with some studies indicating enhanced sensitivity and others indicating increased resistance[74]. For example, one study showed that the p53-deficient cells were sensitized to the effects of DNA-damaging agents as a result of the failure to induce expression of p21, while resistant to the effects of the antimetabolite 5-fluorouracil; p21 was shown to inhibit Cdc2-associated apoptosis[75]. Inappropriate activation of Cdc2 has been implicated or shown to be required for apoptotic cell death[76,77]. In some other systems, however, inactivation of Cdc2 increased the level of apoptosis[78]. The discrepancy regarding Cdc2’s contribution to cell death or survival probably depends on phosphorylation of its downstream targets including BAD and survivin[79-81].
So far, numerous studies addressing the role of E6 in apoptosis have been reported (Figure 2). Since different systems have been used, conflicting and sometimes confusing results have been obtained. As cell types could affect experimental results, we will first focus on apoptosis modulation by E6 in its natural host cells, the keratinocytes or keratinocytes-derived cancer cells. Some interesting observation made in other cell types will be discussed in the third section. For additional information, please see other related reviews[82].
In primary human foreskin keratinocytes, expression of HPV 16 E6 slightly increased spontaneous apoptosis[83,84]. After induction with chemotherapeutic agents such as cisplatin, etoposide, and mitomycin C, enhanced sensitivity in E6 expressing cells was observed[85]. In contrast, E6 inhibited apoptosis during serum- and calcium-induced differentiation of human foreskin keratinocytes[86]. E6 expression correlated with prolonged expression of Bcl-2, reduced elevation of Bax, and loss of p53[86]. While the role of Bcl-2 and Bax in this process remains to be determined, p53 inactivation or E6BP binding do not appear to be essential[87]. Furthermore, co-expression of E6 abrogated E7-mediated apoptosis by TNF[84].
In human keratinocytes immortalized by E6, low levels of apoptosis as compared to the non-immortalized control cells were observed after CD95 (Fas) agonist treatment[88]. Interestingly, in addition to p53 and p21, protein levels of anti-apoptotic proteins Bcl-2 and Flip were reduced. Proteosomal inhibition increased the susceptibility of E6 expressing cells to CD95-mediated apoptosis. But it remains to be determined whether this sensitization is due to increased protein levels of E6, p53, or some other molecules. In another study, E6 reduced UVC-, mitomycin C, and serum starvation-induced apoptosis in the immortalized human keratinocytes (HaCaT) bearing mutated alleles of p53[89,90].
Expression of HPV-16 E6 in HeLa cervical carcinoma cells where the endogenous HPV-18 E6 and E7 transcription were repressed, slightly increased the number of apoptotic cells after prolonged incubation[91]. However, expression of E6 to allow E7 to induce apoptosis is implicated in this study. Similarly, intracellular targeting of HPV-16 E6 by E6-binding polypeptide resulted in apoptosis of HPV-16-positive cervical cancer cells[92]. In contrast, HPV-16 E6 expression in cervical carcinoma C33A cells leads to atractyloside-induced apoptosis[93]. C33A cells do not contain HPV but express mutant p53.
In summary, expression of E6 in primary human keratinocytes or keratinocyte-derived cells consistently induces low level of spontaneous apoptosis. Depending on the agents used, E6 could either sensitize or inhibit keratinocytes to apoptosis after treatment with chemotherapeutic agents.
Numerous studies have been conducted to explore the function of E6 or p53 using cells unrelated to keratinocytes. Different cell types, reagents, and assays were employed. The results are quite inconsistent and sometimes confusing. It is impossible to discuss every report in this review. For this reason, only some representative studies considered to be of special interest will be discussed.
Some early studies showed that E6 inhibited E7-induced apoptosis through p53-independent mechanism in the developing lens of transgenic mice[94,95]. Similarly, E6 could functionally substitute the insulin-like growth factor 1 receptor in inhibiting staurosporine-induced apoptosis in mouse fibroblast, including p53-null cells[96]. Expressing of E6 in human foreskin fibroblasts also inhibited caspase 3 activation after treatment with thiol-containing antioxidant penicillamine[97]. HPV-18 E6 protected cancer cells from Bak-induced apoptosis[98]. E6 of both cutaneous and genital HPVs promoted proteolytic degradation of Bak[61,98]. The role of Bak in UV-induced apoptosis in skin cancer has also been implicated[61].
Several studies have examined the sensitivity of cells expressing E6 to TNF. HPV-16 E6 was shown to bind TNF receptor 1 (TNF R1) and protect cells from TNF-induced apoptosis in mouse fibroblasts and human histiocyte/monocyte and osteosarcoma cells[62,99]. E6 binding to TNF R1 probably interfered with formation of the death-inducing signaling complex and thus with transduction of apoptotic signals. However, E6 did not appear to have much effect on TNF susceptibility in human keratinocytes[84,88]. In contrast, in human ovarian and colon cancer cells, HPV-16 E6 enhanced susceptibility to TNF-induced apoptosis[100]. This effect of E6 appeared to be p53-independent but may involve down-regulation of NF-kappa B. Notably, the BPV-1 E6 oncoprotein sensitized cells to TNF-induced apoptosis[101]. This BPV-1 E6-induced sensitization to apoptosis is distinct from its transforming activity[102]. Interestingly, expression of HPV-16 E6 sensitized murine fibrosarcoma L929 cells to TNF-induced necrosis instead of apoptosis[103]. The E6-enhanced cytolysis correlated with an increase in reactive oxygen species level and was independent of p53 and caspases[103].
In human diploid fibroblasts, expression of HPV-16 E6 resulted in an inhibition of oxidant-induced apoptosis as compared to vector control within 24 h but a sensitization after prolonged incubation[104], indicating that time point at which cell death is measured also contributes to the outcomes. Dying E6 cells exhibited a G2/M phase distribution with elevated cyclin B/Cdc2 levels and activity. The death of E6 cells has some features of oncosis. It remains to be determined to what extent the elevated cyclin B/Cdc2 activity contributes to the cell death in E6-expressing cells. Notably, Normal human fibroblasts expressing HPV-16 E6 showed increased cytotoxicity to taxol[105]. Mutational analysis indicated that reduced levels of p53 correlated with increased G2/M phase arrest and taxol-induced apoptosis[105]. Adriamycin and cisplatin-treated human foreskin fibroblasts expressing E6 also were arrested at G2 with increased cyclin B/Cdc2 kinase activity but no apoptosis[106]. Apparently, activation of cyclin B/Cdc2 kinase in G2/M arrested cells is not by itself sufficient to trigger cell death. E6 expressing cells could also die at other cell cycle stages. For example, when treated with cisplatin, normal human foreskin fibroblasts expressing HPV-16 E6 showed increased cytotoxicity associated with delayed progression through S phase[107].
Progress has been made on observations of E6 regulation of apoptosis. However, the precise mechanism by which E6 modulates apoptosis remains to be explored. In particular, we know little about how E6 sensitizes cells to apoptosis independently of p53. Few studies have addressed the functions of low-risk HPV E6s on cell proliferation and apoptosis. Future studies should also establish the role of more than twenty E6-interacting proteins identified during the past decade. Understanding the mechanism by which E6 regulates apoptosis will certainly help us fully demonstrate the significance of HPV in the etiology of esophageal cancer and possibly have some therapeutic significance.
Co-first-authors: Ting-Ting Li and Li-Na Zhao
| 1. | Schlecht NF, Kulaga S, Robitaille J, Ferreira S, Santos M, Miyamura RA, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2001;286:3106-3114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 361] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Pfister H. Human papillomaviruses and skin cancer. Semin Cancer Biol. 1992;3:263-271. [PubMed] |
| 3. | Syrjänen KJ. Histological changes identical to those of condylomatous lesions found in esophageal squamous cell carcinomas. Arch Geschwulstforsch. 1982;52:283-292. [PubMed] |
| 4. | Winkler B, Capo V, Reumann W, Ma A, La Porta R, Reilly S, Green PM, Richart RM, Crum CP. Human papillomavirus infection of the esophagus. A clinicopathologic study with demonstration of papillomavirus antigen by the immunoperoxidase technique. Cancer. 1985;55:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Awerkiew S, Bollschweiler E, Metzger R, Schneider PM, Hölscher AH, Pfister H. Esophageal cancer in Germany is associated with Epstein-Barr-virus but not with papillomaviruses. Med Microbiol Immunol. 2003;192:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kuwano H, Sumiyoshi K, Sonoda K, Kitamura K, Toh Y, Nakashima H, Sugimachi K. Pathogenesis of esophageal squamous cell carcinoma with lymphoid stroma. Hepatogastroenterology. 2001;48:458-461. [PubMed] |
| 7. | Talamini G, Capelli P, Zamboni G, Mastromauro M, Pasetto M, Castagnini A, Angelini G, Bassi C, Scarpa A. Alcohol, smoking and papillomavirus infection as risk factors for esophageal squamous-cell papilloma and esophageal squamous-cell carcinoma in Italy. Int J Cancer. 2000;86:874-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Kamath AM, Wu TT, Heitmiller R, Daniel R, Shah KV. Investigation of the association of esophageal carcinoma with human papillomaviruses. Dis Esophagus. 2000;13:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Lambot MA, Haot J, Peny MO, Fayt I, Noël JC. Evaluation of the role of human papillomavirus in oesophageal squamous cell carcinoma in Belgium. Acta Gastroenterol Belg. 2000;63:154-156. [PubMed] |
| 10. | Peixoto Guimaraes D, Hsin Lu S, Snijders P, Wilmotte R, Herrero R, Lenoir G, Montesano R, Meijer CJ, Walboomers J, Hainaut P. Absence of association between HPV DNA, TP53 codon 72 polymorphism, and risk of oesophageal cancer in a high-risk area of China. Cancer Lett. 2001;162:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kawaguchi H, Ohno S, Araki K, Miyazaki M, Saeki H, Watanabe M, Tanaka S, Sugimachi K. p53 polymorphism in human papillomavirus-associated esophageal cancer. Cancer Res. 2000;60:2753-2755. [PubMed] |
| 12. | Chang F, Syrjänen S, Shen Q, Cintorino M, Santopietro R, Tosi P, Syrjänen K. Evaluation of HPV, CMV, HSV and EBV in esophageal squamous cell carcinomas from a high-incidence area of China. Anticancer Res. 2000;20:3935-3940. [PubMed] |
| 13. | Chang F, Syrjänen S, Shen Q, Cintorino M, Santopietro R, Tosi P, Syrjänen K. Human papillomavirus involvement in esophageal carcinogenesis in the high-incidence area of China. A study of 700 cases by screening and type-specific in situ hybridization. Scand J Gastroenterol. 2000;35:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Liu YL, Li XM, Jin GL, Yan X, Yang JZ, Wang JL, Li YH, Wang FR, Zhang XH. HPV detection and FHIT expression in esophageal squamous carcinoma from high incidence area in Cixian County. AiZheng. 2003;22:492-495. [PubMed] |
| 15. | Szentirmay Z, Szántó I, Bálint I, Pólus K, Remenár E, Tamás L, Szentkúti G, Melegh Z, Nagy P, Kásler M. Causal association between human papilloma virus infection and head and neck and esophageal squamous cell carcinoma. Magy Onkol. 2002;46:35-41. [PubMed] |
| 16. | Hasegawa M, Ohoka I, Yamazaki K, Hanami K, Sugano I, Nagao T, Asoh A, Wada N, Nagao K, Ishida Y. Expression of p21/WAF-1, status of apoptosis and p53 mutation in esophageal squamous cell carcinoma with HPV infection. Pathol Int. 2002;52:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Matsha T, Erasmus R, Kafuko AB, Mugwanya D, Stepien A, Parker MI. Human papillomavirus associated with oesophageal cancer. J Clin Pathol. 2002;55:587-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Astori G, Merluzzi S, Arzese A, Brosolo P, de Pretis G, Maieron R, Pipan C, Botta GA. Detection of human papillomavirus DNA and p53 gene mutations in esophageal cancer samples and adjacent normal mucosa. Digestion. 2001;64:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Lu Z, Chen K, Guo M. Detection of HPV in human esophageal cancer in high-incidence area and its correlation with p53 expression. Zhonghua ZhongLiu ZaZhi. 2001;23:220-223. [PubMed] |
| 20. | Zhou XB, Guo M, Quan LP, Zhang W, Lu ZM, Wang QH, Ke Y, Xu NZ. Detection of human papillomavirus in Chinese esophageal squamous cell carcinoma and its adjacent normal epithelium. World J Gastroenterol. 2003;9:1170-1173. [PubMed] |
| 21. | Shen ZY, Hu SP, Lu LC, Tang CZ, Kuang ZS, Zhong SP, Zeng Y. Detection of human papillomavirus in esophageal carcinoma. J Med Virol. 2002;68:412-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Xu WG, Zhang LJ, Lu ZM, Li JY, Ke Y, Xu GW. Detection of human papillomavirus type 16 E6 mRNA in carcinomas of upper digestive tract. Zhonghua YiXue ZaZhi. 2003;83:1910-1914. [PubMed] |
| 23. | Sobti RC, Kochar J, Singh K, Bhasin D, Capalash N. Telomerase activation and incidence of HPV in human gastrointestinal tumors in North Indian population. Mol Cell Biochem. 2001;217:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Xu CL, Qian XL, Zhou XS, Zhao QZ, Li YC. Expression of HPV16-E6 and E7 oncoproteins in squamous cell carcinoma tissues of esophageal cancer and non-cancer tissues. AiZheng. 2004;23:165-168. [PubMed] |
| 25. | Acevedo-Nuño E, González-Ojeda A, Vázquez-Camacho G, Balderas-Peña Luz Ma A, Moreno-Villa H, Montoya-Fuentes H. Human papillomavirus DNA and protein in tissue samples of oesophageal cancer, Barrett's oesophagus and oesophagitis. Anticancer Res. 2004;24:1319-1323. [PubMed] |
| 26. | Li T, Lu ZM, Chen KN, Guo M, Xing HP, Mei Q, Yang HH, Lechner JF, Ke Y. Human papillomavirus type 16 is an important infectious factor in the high incidence of esophageal cancer in Anyang area of China. Carcinogenesis. 2001;22:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Togawa K, Rustgi AK. Human papillomavirus-16 and -18 replication in esophagus squamous cancer cell lines does not require heterologous E1 and E2 proteins. J Med Virol. 1995;45:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Jarrett WF, McNeil PE, Grimshaw WT, Selman IE, McIntyre WI. High incidence area of cattle cancer with a possible interaction between an environmental carcinogen and a papilloma virus. Nature. 1978;274:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 173] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Shen Z, Cen S, Shen J, Cai W, Xu J, Teng Z, Hu Z, Zeng Y. Study of immortalization and malignant transformation of human embryonic esophageal epithelial cells induced by HPV18 E6E7. J Cancer Res Clin Oncol. 2000;126:589-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Shen ZY, Xu LY, Li EM, Cai WJ, Shen J, Chen MH, Cen S, Tsao SW, Zeng Y. The multistage process of carcinogenesis in human esophageal epithelial cells induced by human papillomavirus. Oncol Rep. 2004;11:647-654. [PubMed] |
| 32. | Omata M, Mori J, Yokosuka O, Iwama S, Ito Y, Okuda K. Hepatitis B virus antigens in liver tissue in hepatocellular carcinoma and advanced chronic liver disease-relationship to liver cell dysplasia. Liver. 1982;2:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1039] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 34. | Alvarez-Salas LM, Cullinan AE, Siwkowski A, Hampel A, DiPaolo JA. Inhibition of HPV-16 E6/E7 immortalization of normal keratinocytes by hairpin ribozymes. Proc Natl Acad Sci USA. 1998;95:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Barbosa MS, Lowy DR, Schiller JT. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989;63:1404-1407. [PubMed] |
| 36. | Grossman SR, Laimins LA. E6 protein of human papillomavirus type 18 binds zinc. Oncogene. 1989;4:1089-1093. [PubMed] |
| 37. | Kanda T, Watanabe S, Zanma S, Sato H, Furuno A, Yoshiike K. Human papillomavirus type 16 E6 proteins with glycine substitution for cysteine in the metal-binding motif. Virology. 1991;185:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Vousden KH, Androphy EJ, Schiller JT, Lowy DR. Mutational analysis of bovine papillomavirus E6 gene. J Virol. 1989;63:2340-2342. [PubMed] |
| 39. | Lamberti C, Morrissey LC, Grossman SR, Androphy EJ. Transcriptional activation by the papillomavirus E6 zinc finger oncoprotein. EMBO J. 1990;9:1907-1913. [PubMed] |
| 40. | Foster SA, Demers GW, Etscheid BG, Galloway DA. The ability of human papillomavirus E6 proteins to target p53 for degradation in vivo correlates with their ability to abrogate actinomycin D-induced growth arrest. J Virol. 1994;68:5698-5705. [PubMed] |
| 41. | Dalal S, Gao Q, Androphy EJ, Band V. Mutational analysis of human papillomavirus type 16 E6 demonstrates that p53 degradation is necessary for immortalization of mammary epithelial cells. J Virol. 1996;70:683-688. [PubMed] |
| 42. | Ned R, Allen S, Vande Pol S. Transformation by bovine papillomavirus type 1 E6 is independent of transcriptional activation by E6. J Virol. 1997;71:4866-4870. [PubMed] |
| 43. | Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo. J Virol. 2003;77:6957-6964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Watson RA, Thomas M, Banks L, Roberts S. Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J Cell Sci. 2003;116:4925-4934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Kühne C, Gardiol D, Guarnaccia C, Amenitsch H, Banks L. Differential regulation of human papillomavirus E6 by protein kinase A: conditional degradation of human discs large protein by oncogenic E6. Oncogene. 2000;19:5884-5891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Androphy EJ, Hubbert NL, Schiller JT, Lowy DR. Identification of the HPV-16 E6 protein from transformed mouse cells and human cervical carcinoma cell lines. EMBO J. 1987;6:989-992. [PubMed] |
| 47. | Guccione E, Massimi P, Bernat A, Banks L. Comparative analysis of the intracellular location of the high- and low-risk human papillomavirus oncoproteins. Virology. 2002;293:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Le Roux LG, Moroianu J. Nuclear entry of high-risk human papillomavirus type 16 E6 oncoprotein occurs via several pathways. J Virol. 2003;77:2330-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Mallon RG, Wojciechowicz D, Defendi V. DNA-binding activity of papillomavirus proteins. J Virol. 1987;61:1655-1660. [PubMed] |
| 50. | Kämmer C, Warthorst U, Torrez-Martinez N, Wheeler CM, Pfister H. Sequence analysis of the long control region of human papillomavirus type 16 variants and functional consequences for P97 promoter activity. J Gen Virol. 2000;81:1975-1981. [PubMed] |
| 51. | Nominé Y, Charbonnier S, Ristriani T, Stier G, Masson M, Cavusoglu N, Van Dorsselaer A, Weiss E, Kieffer B, Travé G. Domain substructure of HPV E6 oncoprotein: biophysical characterization of the E6 C-terminal DNA-binding domain. Biochemistry. 2003;42:4909-4917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Ristriani T, Masson M, Nominé Y, Laurent C, Lefevre JF, Weiss E, Travé G. HPV oncoprotein E6 is a structure-dependent DNA-binding protein that recognizes four-way junctions. J Mol Biol. 2000;296:1189-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874-7887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 364] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 54. | Flores ER, Allen-Hoffmann BL, Lee D, Lambert PF. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J Virol. 2000;74:6622-6631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 55. | Münger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 525] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 56. | Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129-4135. [PubMed] |
| 57. | Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1751] [Cited by in RCA: 1806] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 58. | el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6088] [Cited by in RCA: 6307] [Article Influence: 197.1] [Reference Citation Analysis (0)] |
| 59. | Burkhart BA, Alcorta DA, Chiao C, Isaacs JS, Barrett JC. Two posttranscriptional pathways that regulate p21(Cip1/Waf1/Sdi1) are identified by HPV16-E6 interaction and correlate with life span and cellular senescence. Exp Cell Res. 1999;247:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Giannoudis A, Herrington CS. Differential expression of p53 and p21 in low grade cervical squamous intraepithelial lesions infected with low, intermediate, and high risk human papillomaviruses. Cancer. 2000;89:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14:3065-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 235] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 62. | Filippova M, Song H, Connolly JL, Dermody TS, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells from TNF-induced apoptosis. J Biol Chem. 2002;277:21730-21739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Nagata S. Apoptosis by death factor. Cell. 1997;88:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3739] [Cited by in RCA: 3644] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 64. | Wyllie AH, Golstein P. More than one way to go. Proc Natl Acad Sci USA. 2001;98:11-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Irusta PM, Chen YB, Hardwick JM. Viral modulators of cell death provide new links to old pathways. Curr Opin Cell Biol. 2003;15:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 343] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 67. | Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5324] [Cited by in RCA: 5482] [Article Influence: 195.8] [Reference Citation Analysis (0)] |
| 68. | Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2314] [Cited by in RCA: 2233] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 69. | Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2665] [Cited by in RCA: 2647] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 70. | Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3283] [Cited by in RCA: 3288] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 71. | Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2420] [Cited by in RCA: 2427] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 72. | Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1960] [Cited by in RCA: 2297] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 73. | Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1662] [Cited by in RCA: 1760] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 74. | Blandino G, Levine AJ, Oren M. Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene. 1999;18:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 75. | Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 812] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 76. | Shi L, Nishioka WK, Th'ng J, Bradbury EM, Litchfield DW, Greenberg AH. Premature p34cdc2 activation required for apoptosis. Science. 1994;263:1143-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 432] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 77. | Tan M, Jing T, Lan KH, Neal CL, Li P, Lee S, Fang D, Nagata Y, Liu J, Arlinghaus R. Phosphorylation on tyrosine-15 of p34(Cdc2) by ErbB2 inhibits p34(Cdc2) activation and is involved in resistance to taxol-induced apoptosis. Mol Cell. 2002;9:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Badie C, Itzhaki JE, Sullivan MJ, Carpenter AJ, Porter AC. Repression of CDK1 and other genes with CDE and CHR promoter elements during DNA damage-induced G(2)/M arrest in human cells. Mol Cell Biol. 2000;20:2358-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, Altieri DC. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci USA. 2000;97:13103-13107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 497] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 80. | Wall NR, O'Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63:230-235. [PubMed] |
| 81. | Konishi Y, Lehtinen M, Donovan N, Bonni A. Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell. 2002;9:1005-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 223] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 82. | Finzer P, Aguilar-Lemarroy A, Rösl F. The role of human papillomavirus oncoproteins E6 and E7 in apoptosis. Cancer Lett. 2002;188:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Iglesias M, Yen K, Gaiotti D, Hildesheim A, Stoler MH, Woodworth CD. Human papillomavirus type 16 E7 protein sensitizes cervical keratinocytes to apoptosis and release of interleukin-1alpha. Oncogene. 1998;17:1195-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Stöppler H, Stöppler MC, Johnson E, Simbulan-Rosenthal CM, Smulson ME, Iyer S, Rosenthal DS, Schlegel R. The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene. 1998;17:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 85. | Liu Y, McKalip A, Herman B. Human papillomavirus type 16 E6 and HPV-16 E6/E7 sensitize human keratinocytes to apoptosis induced by chemotherapeutic agents: roles of p53 and caspase activation. J Cell Biochem. 2000;78:334-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 86. | Alfandari J, Shnitman Magal S, Jackman A, Schlegel R, Gonen P, Sherman L. HPV16 E6 oncoprotein inhibits apoptosis induced during serum-calcium differentiation of foreskin human keratinocytes. Virology. 1999;257:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Sherman L, Itzhaki H, Jackman A, Chen JJ, Koval D, Schlegel R. Inhibition of serum- and calcium-induced terminal differentiation of human keratinocytes by HPV 16 E6: study of the association with p53 degradation, inhibition of p53 transactivation, and binding to E6BP. Virology. 2002;292:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Aguilar-Lemarroy A, Gariglio P, Whitaker NJ, Eichhorst ST, zur Hausen H, Krammer PH, Rösl F. Restoration of p53 expression sensitizes human papillomavirus type 16 immortalized human keratinocytes to CD95-mediated apoptosis. Oncogene. 2002;21:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Magal SS, Jackman A, Pei XF, Schlegel R, Sherman L. Induction of apoptosis in human keratinocytes containing mutated p53 alleles and its inhibition by both the E6 and E7 oncoproteins. Int J Cancer. 1998;75:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 90. | Mythily DV, Krishna S, Tergaonkar V. Pleiotropic effects of human papillomavirus type 16 E6 oncogene expression in human epithelial cell lines. J Gen Virol. 1999;80:1707-1713. [PubMed] |
| 91. | DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol. 2003;77:1551-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 92. | Butz K, Denk C, Ullmann A, Scheffner M, Hoppe-Seyler F. Induction of apoptosis in human papillomaviruspositive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc Natl Acad Sci USA. 2000;97:6693-6697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Brown J, Higo H, McKalip A, Herman B. Human papillomavirus (HPV) 16 E6 sensitizes cells to atractyloside-induced apoptosis: role of p53, ICE-like proteases and the mitochondrial permeability transition. J Cell Biochem. 1997;66:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Pan H, Griep AE. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994;8:1285-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 296] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 95. | Pan H, Griep AE. Temporally distinct patterns of p53-dependent and p53-independent apoptosis during mouse lens development. Genes Dev. 1995;9:2157-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 158] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 96. | Steller MA, Zou Z, Schiller JT, Baserga R. Transformation by human papillomavirus 16 E6 and E7: role of the insulin-like growth factor 1 receptor. Cancer Res. 1996;56:5087-5091. [PubMed] |
| 97. | Havre PA, O'Reilly S, McCormick JJ, Brash DE. Transformed and tumor-derived human cells exhibit preferential sensitivity to the thiol antioxidants, N-acetyl cysteine and penicillamine. Cancer Res. 2002;62:1443-1449. [PubMed] |
| 98. | Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene. 1998;17:2943-2954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 207] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Duerksen-Hughes PJ, Yang J, Schwartz SB. HPV 16 E6 blocks TNF-mediated apoptosis in mouse fibroblast LM cells. Virology. 1999;264:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Vikhanskaya F, Falugi C, Valente P, Russo P. Human papillomavirus type 16 E6-enhanced susceptibility to apoptosis induced by TNF in A2780 human ovarian cancer cell line. Int J Cancer. 2002;97:732-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Rapp L, Liu Y, Hong Y, Androphy EJ, Chen JJ. The bovine papillomavirus type 1 E6 oncoprotein sensitizes cells to tumor necrosis factor alpha-induced apoptosis. Oncogene. 1999;18:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 102. | Liu Z, Liu Y, Hong Y, Rapp L, Androphy EJ, Chen JJ. Bovine papillomavirus type 1 E6-induced sensitization to apoptosis is distinct from its transforming activity. Virology. 2002;295:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 103. | Liu Y, Tergaonkar V, Krishna S, Androphy EJ. Human papillomavirus type 16 E6-enhanced susceptibility of L929 cells to tumor necrosis factor alpha correlates with increased accumulation of reactive oxygen species. J Biol Chem. 1999;274:24819-24827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Chen X, Shen B, Xia L, Khaletzkiy A, Chu D, Wong JY, Li JJ. Activation of nuclear factor kappaB in radioresistance of TP53-inactive human keratinocytes. Cancer Res. 2002;62:1213-1221. [PubMed] |
| 105. | Wahl AF, Donaldson KL, Fairchild C, Lee FY, Foster SA, Demers GW, Galloway DA. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat Med. 1996;2:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 458] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 106. | Passalaris TM, Benanti JA, Gewin L, Kiyono T, Galloway DA. The G(2) checkpoint is maintained by redundant pathways. Mol Cell Biol. 1999;19:5872-5881. [PubMed] |
| 107. | Hawkins DS, Demers GW, Galloway DA. Inactivation of p53 enhances sensitivity to multiple chemotherapeutic agents. Cancer Res. 1996;56:892-898. [PubMed] |