Published online Feb 21, 2005. doi: 10.3748/wjg.v11.i7.1060
Revised: June 19, 2004
Accepted: July 15, 2004
Published online: February 21, 2005
AIM: To investigate the effect of tamoxifen (TAM) on multidrug resistance (MDR) of colorectal carcinoma in vivo and its relationship with estrogen receptor (ER).
METHODS: Multidrug resistance was determined by means of semi-quantitative retro-transcription polymerase chain reaction (RT-PCR) to test mdr1 gene mRNA and ER expression was studied by immunohistochemistry. Tumor tissues from three cases of human colon carcinoma, which had mdr1(+)/ER(+), mdr1(+)/ER(-), mdr1(-) expressions, were planted subcutaneously in the neck of nude mice to establish three xenograft models. These models were subdivided into four subgroups randomly: Doxorubicin (DOX)-treated group, TAM-treated group, DOX and TAM group and control group. The dimensions of these xenografts were measured after each course of treatment and the xenografts were removed at the end of the experiments for measurements of weight and the variation of mdr1 mRNA level with RT-PCR. In each course, TAM [15 mg/(kg/d)] was administrated orally per day in the first seven days and DOX (3.6 mg/kg) was injected peritoneally on the first day. Data was evaluated by q and t tests.
RESULTS: In the animal models with mdr1(-) tumor, the weights and volumes of the planted tumor in DOX group [(39.1±2.29) mg, (31.44±1.61) mm3] and TAM and DOX group [(38.72±2.56) mg, (31.31±1.74) mm3], which were lesser than that of control group [(45.48±3.92) mg, (36.42±2.77) mm3, P = 0.037, P = 0.016 respectively] significantly. In the animal models with mdr1(+)/ER(+) tumor, the weights and volumes of planted tumor were not affected by DOX or TAM treatment; however, in TAM and DOX group [(425.5±28.58) mg, (340.35±22.28) mm3], they were significantly less than that of control group [(634.23±119.41) mg, (507.45±93.34) mm3, P = 0.022, P = 0.045 respectively], which are similar to that in the models with mdr1(+)/ER(-) tumor. No significant changes were found in the expressive level of mdr1 mRNA following these treatments.
CONCLUSION: The expression of mdr1 gene corresponds to the sensitivity of colon cancer to anti-tumor drugs in vivo. TAM can reverse the MDR of colorectal carcinoma in nude mice, which is independent of the expression of ER; however, no change was observed in the expressive level of mdr1 mRNA.
-
Citation: Shen LZ, Hua YB, Yu XM, Xu Q, Chen T, Wang JH, Wu WX. Tamoxifen can reverse multidrug resistance of colorectal carcinoma
in vivo . World J Gastroenterol 2005; 11(7): 1060-1064 - URL: https://www.wjgnet.com/1007-9327/full/v11/i7/1060.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i7.1060
Multidrug resistance (MDR) describes the cross-resistance to various structurally unrelated cytotoxic agents in human neoplasms. Resistance to chemotherapy remains an obstacle to the successful treatment of human cancer and has been the subject of numerous investigations aiming at identifying the molecular mechanisms of resistance in cancer cells[1]. It has been shown that MDR has many mechanisms, including the over-expression of mdr1 gene, the enhancement of multidrug-resistant protein and lung-resistant protein, the variation of DNA topoisomerase II and glutathione-S-transferase (GST). But mdr1 over expression is being regarded as the major factor[2-4]. The mdr1 gene encodes a transmembrane protein, called P-glycoprotein (P-gp), which acts as a drug efflux pump actively depleting intracellular drug concentration in resistant tumor cells. The colorectal carcinoma has been estimated as one of the tumors with the highest expression of mdr1 gene or P-gp.
Experimentally, MDR can be reversed completely or partly by simultaneous treatment with a number of non-cytotoxic agents, which can competitively inhibit P-gp function[5-9]. These agents include tamoxifen (TAM), cyclosporin A (CsA), verapamil (VER), BSO, etc. It has been estimated that the MDR of colorectal cells can be reversed by administration of TAM in vitro, which has not been shown in vivo. It has been repeatedly demonstrated that estrogen has a close bearing on the carcinogenesis of estrogen receptor (ER) containing target organs such as uterus, vagina and breast. ER was also found in both normal and neoplastic colorectal epithelia[10]. TAM has been used widely in the treatment of advanced breast cancer, with high responses in patients with positive expression of ER. In this study, xenograft models of nude mice with human colorectal carcinoma were established to determine whether TAM had the same effect in vivo as in vitro on MDR and its relationship with ER.
Semi-quantitative RT-PCR assay to test mRNA and immunohistochemistry assay to examine ER expression are described in our previous report[11].
BALB/c (nu/nu) nude mice, four to six months old, were purchased from Shanghai Institute of Experimental Animals, Chinese Academy of Sciences, and bred in SPF environment in Jiangsu Experimental Animal Center. These nude mice were experimented irrespective of their genders.
Human colorectal carcinoma tissue was obtained during operation aseptically and was broken into pieces as small as possible, and then this tissue was transplanted into nude mice subcutaneously. It was required that tumor tissue be transplanted in thirty minutes after resection and transplantation operation be sterile. About four weeks later at the transplantation site tumor appeared and grew large gradually. When the tumor in nude mice grew to about 2-3 centimeters in diameter, it was taken out and cut into one square millimeter pieces and each piece planted into nude mice to establish the second-generation model, which was used in this study.
Before we established the nude mouse models, we had done some biopsies by electronic colonoscopic examination to screen out three patients with colorectal carcinoma, whose expressions of ER and mdr1 gene were ER positive/mdr1 positive, ER negative/mdr1 positive, mdr1 negative respectively. Patient I, male, with both ER and mdr1 positive, was thirty-six years old and had poorly differentiated carcinoma in ascending colon, which was Duke’s stage B. Patient II, male, with ER negativity but mdr1 positivity, was forty-three years old and had poorly differentiated carcinoma in sigmoid colon, which was Duke’s stage C. Patient III, female, mdr1 negative, had well-differentiated carcinoma in sigmoid colon, which was also Duke’s stage C.
The second-generation models from one patient were divided into four groups randomly: DOX-treated group, TAM-treated group, DOX and TAM group and control group. Each group contained six nude mice.
TAM (Shanghai Hualian Pharmaceutical Co. Ltd) was dissolved in normal saline and administered orally at 15 mg/kg body weight per day. DOX (Shanghai Hualian Pharmaceutical Co. Ltd) was injected into peritoneal cavity at 3.6 mg/kg body weight.
TAM was administered orally every day in the first seven days in TAM-treated groups and in TAM and DOX groups. DOX was injected on the first day in DOX-treated groups and in TAM and DOX groups. There was no administration during the eighth to the twenty-first days. There were two courses totally.
At the beginning and end of each course, the dimensions of these tumors were measured (X stands for the maximal length and Y for the minimal length), and then tumor volumes were calculated by the formula, V = 0.4xy2. By the end of this experiment, the tumors were taken out and weighed, and the mdr1 mRNA levels of these treated tumors were evaluated again.
The volumes and weights of the tumors were compared among different groups by q test, so were the mdr1 RNA levels between pre- and post- experiment using t test.
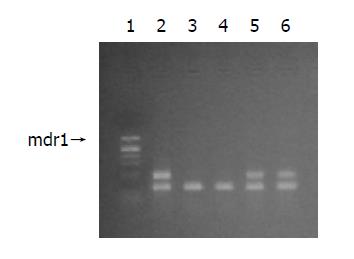
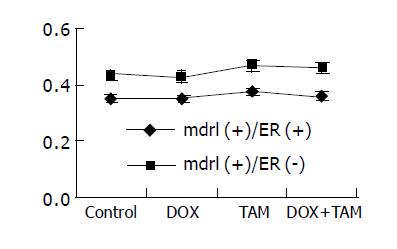
Figure 1 shows the results of mdr1 mRNA expression before experiments.
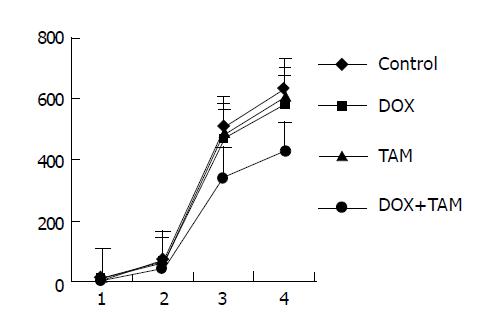
The volumes and weights of mdr1-positive and ER-positive tumors are shown in Figure 2. By the end of the first course the volumes of tumors were nearly equal among the control group [(62.63±10.16) cm3], DOX-treated group [(57.58±8.36) cm3] and TAM-treated group [(59.22±8.53) cm3], but the tumors in DOX+TAM group [(44.37±4.02) cm3] were smaller than those of control group (P = 0.031). Similarly, at the end of treatment the tumor volumes and weights of TAM+DOX group [(340.35±22.28) cm3, (425.52±28.58) mg] were smaller than those of control group significantly [(507.45±93.34) cm3, (634.23±119.41) mg, P = 0.022, P = 0.045 respectively]; both the tumors in TAM-treated group [(483.68±81.96) cm3, (603.05±103.39) mg] and DOX-treated group [(465.92± 72.94) cm3, (581.05±89.77) mg] were similar to those of control group in diameter.
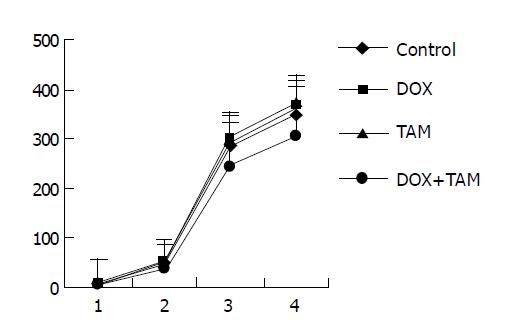
The result of mdr1-positive and ER-negative group is shown in Figure 3. Similar to those of both mdr1 and ER-positive tumors, at the end of first and second courses of treatment the tumors in TAM+DOX group were smaller than those of control group significantly (P = 0.040, P = 0.001 respectively); however, the tumors in TAM-treated group and DOX-treated group were relatively equal to those of control group.
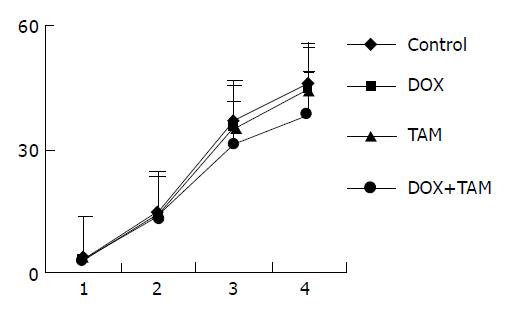
The result of mdr1-negative group is shown in Figure 4. The tumors were nearly equal among four groups after the first course, but by the end of treatment the tumors in TAM+DOX group [(31.31±1.74) cm3, (38.72±2.56) mg] and DOX group [(31.44±1.61) cm3, (39.1±2.29) mg] were smaller than those of control group [(36.42±2.77) cm3, (45.48±3.92) mg] and TAM group [(35.47±3.5) cm3, (44.57±3.74) mg] significantly (P = 0.037, P = 0.016 respectively).
The ratio of mdr1 mRNA/β2MG mRNA of tumor from patient I (both mdr1 and ER positive) was 0.36, and from patient II (ER negative but mdr1 positive) 0.44 before treatment. The results after treatment are shown in Figure 5. Obviously, the mdr1 mRNA levels of these tumors remained unchanged after treatment.
Multidrug resistance (MDR) was first observed in experimental oncology in 1970 by Biedler and Riehm. It has been extensively studied because one of the most serious problems in current cancer chemotherapy is intrinsic or acquired drug resistance. P-glycoprotein (P-gp) encoded by mdr1 gene, is believed to play an important role in the mechanism of MDR, although the latter involves many factors. P-gp is a membrane ATPase that serves as an efflux pump for multiple anticancer agents. This protein has 12 transmembrane domains divided into two homologous halves, each of which includes an ATP-binding cassette domain that catalyzes ATP hydrolysis. P-gp is found in normal bile duct, pancreatic duct, small intestine and large intestine, although its amount is very low. P-gp in normal tissue is thought to serve some functions such as withstanding the invasion of exotic toxin, excreting by-product of metabolism or several kinds of cytokine via non-typical pathway, transferring ions and hormones.
Colorectal cancer is one of the tumors with the highest expression of mdr1 gene or P-gp. Previously, we showed by immunohistochemistry analysis that 42.9% normal colorectal specimens expressed P-gp; however, in untreated colorectal tumors P-gp expression rate was 73.85%, which was significantly higher than that in the corresponding normal tissue, indicating the colon epithelium, which acquired high expression of P-gp/mdr1 in the course of carcinogenesis[11]. As to the clinical relationship between overexpression of P-gp/mdr1 with multiple drug resistance, numerous researches have demonstrated the direct relevancy[3,4,12]. In the current research, tumors with negative mdr1 expression were smaller than those in control groups after DOX treatment, but DOX did not influence the growth of mdr1-positive tumors, that is to say, tumors with increased mdr1 gene expression showed resistance to DOX, confirming the relationship between mdr1 gene and anticancer drug resistance in nude mouse models.
The relationship between hormone and neoplasm is relatively complicated[13]. It has been repeatedly demonstrated that estrogen has a close bearing on the carcinogenesis of its estrogen receptor (ER)-containing target organs such as uterus, vagina and breast. Estrogen functions through integrating with estrogen receptor (ER), but ER protein was also found in both normal and neoplastic colorectal epithelium, which was firstly reported by Jensen and his colleagues. The expression rate of ER in primary colorectal carcinoma varied widely from 20% to 80% according to different reports[10,14-20]. In our previous study, we demonstrated ER expression in colorectal carcinomas and adjacent normal mucosa with expression rates of 75.4% and 38.1% respectively[11]. ER expression in carcinomas was more extensive than in normal tissues indicating that during the course of carcinogenesis colorectal epithelium obtained high expression of ER and estrogen might play some role in differentiation and maturation of colorectal cells, which is different from results of Konstantinopoulos et al[21]. Previously, there was no report on co-expression of ER and P-gp; however, the positive relevancy between the expression of ER and P-gp was not found in our study.
Anti-estrogen tamoxifen (TAM) is one of the compounds that can modify multidrug resistance. It is widely used in the treatment of advanced breast cancers with a high response in tumors containing ER. Despite numerous in vitro studies and clinical trials of TAM having been conducted, it is necessary to investigate its effect in vivo and its relationship with ER status. In the current research, three colorectal cancer specimens from different patients with different expression of ER or mdr1 gene were used to establish xenograft models in nude mice, which ensured the results more close to clinical study.
Although Nakayama’s research[22] showed ERbeta ligands in combination with tamoxifen may have tumor-static effects on colon cancer cells, our results showed that only tamoxifen did not influence the growth of tumors despite several kinds of expressions of ER and mdr1 gene, indicating that only TAM may have no effect on colon cancer. However, in tumors with mdr1 gene, TAM did inhibit tumors significantly irrespective of ER status when it was administered with DOX, that is to say, TAM can modify multidrug resistance, and its effect is independent of estrogen receptor. It was also demonstrated that the mdr1 gene levels in these tumors were not influenced by TAM and DOX treatment. The mechanism of TAM to reverse MDR requires further investigation. The dose of TAM used in this study was too high for humans, so its innate toxicity may prevent it from being used in humans as a potent modulating agent. Other chemical compounds also produce undesirable side effects in vivo, such as cardiovascular disorders.
Recent advances in molecular technology make it possible to introduce genetic modification of the cells to the field of treatment[23-27]. Reports have already described that treatment of anti-sense oligonucleotides against P-gp[23] and P-gp ribozyme[24] overcame MDR and enhanced the chemosensitivity of treated cells. Heike et al[28] used an intracellular anti-mdr1 sFv to overcome MDR, and Zhan et al[29] found reintroduction of wt p53 into soft tissue sarcoma cells harboring p53 mutations enhanced their chemosensitivity to DOX through the inhibition of mdr1/P-gp expression.
Co-first-author: Yi-Bing Hua
| 1. | Yin F, Shi YQ, Chen CP, Qiao TD, Chen BJ, Miao JY, Fan DM. Expression of cDNA fragment encoding MGr1-Ag1 detected by MDR related antibody MGr1 in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2003;11:18-21. |
| 2. | Uchiyama-Kokubu N, Watanabe T. Establishment and characterization of adriamycin-resistant human colorectal adenocarcinoma HCT-15 cell lines with multidrug resistance. Anticancer Drugs. 2001;12:769-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Wang BL, Zhai HY, Chen BY, Zhai SP, Yang HY, Chen XP, Zhao WT, Meng L. Clinical relationship between MDR1 gene and gallbladder cancer. Hepatobiliary Pancreat Dis Int. 2004;3:296-299. [PubMed] |
| 4. | Lorke DE, Krüger M, Buchert R, Bohuslavizki KH, Clausen M, Schumacher U. In vitro and in vivo tracer characteristics of an established multidrug-resistant human colon cancer cell line. J Nucl Med. 2001;42:646-654. [PubMed] |
| 5. | Tu SP, Jiang SH, Qiao MM, Cheng SD, Wang LF, Wu YL, Yuan YZ, Wu YX. Effect of trichosanthin on cytotoxicity and induction of apoptosis of multiple drugs resistence cells in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:150-152. |
| 6. | Molnar J, Mucsi I, Nacsa J, Hevér A, Gyémánt N, Ugocsai K, Hegyes P, Kiessig S, Gaal D, Lage H. New silicon compounds as resistance modifiers against multidrug-resistant cancer cells. Anticancer Res. 2004;24:865-871. [PubMed] |
| 7. | Madureira AM, Spengler G, Molnár A, Varga A, Molnár J, Abreu PM, Ferreira MJ. Effect of cycloartanes on reversal of multidrug resistance and apoptosis induction on mouse lymphoma cells. Anticancer Res. 2004;24:859-864. [PubMed] |
| 8. | Brooks TA, Kennedy DR, Gruol DJ, Ojima I, Baer MR, Bernacki RJ. Structure-activity analysis of taxane-based broad-spectrum multidrug resistance modulators. Anticancer Res. 2004;24:409-415. [PubMed] |
| 9. | Bergmann R, Brust P, Scheunemann M, Pietzsch HJ, Seifert S, Roux F, Johannsen B. Assessment of the in vitro and in vivo properties of a (99m)Tc-labeled inhibitor of the multidrug resistant gene product P-glycoprotein. Nucl Med Biol. 2000;27:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Picariello L, Fiorelli G, Martineti V, Tognarini I, Pampaloni B, Tonelli F, Brandi ML. Growth response of colon cancer cell lines to selective estrogen receptor modulators. Anticancer Res. 2003;23:2419-2424. [PubMed] |
| 11. | Shen LZ, Wu WX. Expression of P-glycoprotein and estrogen receptor in human primary colorectal carcinoma. J Nanjing Med University. 1999;13:61-69. |
| 12. | Meschini S, Calcabrini A, Monti E, Del Bufalo D, Stringaro A, Dolfini E, Arancia G. Intracellular P-glycoprotein expression is associated with the intrinsic multidrug resistance phenotype in human colon adenocarcinoma cells. Int J Cancer. 2000;87:615-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39:1251-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 250] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Di Leo A, Messa C, Cavallini A, Linsalata M. Estrogens and colorectal cancer. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | al-Azzawi F, Wahab M. Estrogen and colon cancer: current issues. Climacteric. 2002;5:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Witte D, Chirala M, Younes A, Li Y, Younes M. Estrogen receptor beta is expressed in human colorectal adenocarcinoma. Hum Pathol. 2001;32:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632-640. [PubMed] |
| 18. | Arai N, Ström A, Rafter JJ, Gustafsson JA. Estrogen receptor beta mRNA in colon cancer cells: growth effects of estrogen and genistein. Biochem Biophys Res Commun. 2000;270:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Slattery ML, Samowitz WS, Holden JA. Estrogen and progesterone receptors in colon tumors. Am J Clin Pathol. 2000;113:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000;60:245-248. [PubMed] |
| 21. | Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour's dedifferentiation. Eur J Cancer. 2003;39:1251-1258. |
| 22. | Nakayama Y, Sakamoto H, Satoh K, Yamamoto T. Tamoxifen and gonadal steroids inhibit colon cancer growth in association with inhibition of thymidylate synthase, survivin and telomerase expression through estrogen receptor beta mediated system. Cancer Lett. 2000;161:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Ramachandran C, Wellham LL. Effect of MDR1 phosphorothioate antisense oligodeoxynucleotides in multidrug-resistant human tumor cell lines and xenografts. Anticancer Res. 2003;23:2681-2690. [PubMed] |
| 24. | Wang H, Chen XP, Qiu FZ. Overcoming multi-drug resistance by anti-MDR1 ribozyme. World J Gastroenterol. 2003;9:1444-1449. [PubMed] |
| 25. | Kang CD, Ahn BK, Jeong CS, Kim KW, Lee HJ, Yoo SD, Chung BS, Kim SH. Downregulation of JNK/SAPK activity is associated with the cross-resistance to P-glycoprotein-unrelated drugs in multidrug-resistant FM3A/M cells overexpressing P-glycoprotein. Exp Cell Res. 2000;256:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Nagata J, Kijima H, Hatanaka H, Asai S, Miyachi H, Abe Y, Yamazaki H, Nakamura M, Watanabe N, Mine T. Reversal of drug resistance using hammerhead ribozymes against multidrug resistance-associated protein and multidrug resistance 1 gene. Int J Oncol. 2002;21:1021-1026. [PubMed] |
| 27. | Nagata J, Kijima H, Hatanaka H, Asai S, Miyachi H, Takagi A, Miwa T, Mine T, Yamazaki H, Nakamura M. Reversal of cisplatin and multidrug resistance by ribozyme-mediated glutathione suppression. Biochem Biophys Res Commun. 2001;286:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Heike Y, Kasono K, Kunisaki C, Hama S, Saijo N, Tsuruo T, Kuntz DA, Rose DR, Curiel DT. Overcoming multi-drug resistance using an intracellular anti-MDR1 sFv. Int J Cancer. 2001;92:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Zhan M, Yu D, Lang A, Li L, Pollock RE. Wild type p53 sensitizes soft tissue sarcoma cells to doxorubicin by down-regulating multidrug resistance-1 expression. Cancer. 2001;92:1556-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |