Published online Feb 14, 2005. doi: 10.3748/wjg.v11.i6.903
Revised: September 12, 2004
Accepted: October 8, 2004
Published online: February 14, 2005
AIM: To investigate whether NF-κB is activated in human gastric carcinoma tissues and, if so, to study whether there is any correlation between NF-κB activity and heparanase expression in gastric carcinoma.
METHODS: NF-κB activation was assayed by immunohi-stochemical staining in formalin-fixed, paraffin-embedded specimens from 45 gastric carcinoma patients. Electrophoretic mobility shift assay (EMSA) method was used for nuclear protein from these fresh tissue specimens. Heparanase gene expression was quantified using quantitative RT-PCR.
RESULTS: The nuclear translocation of RelA (marker of NF-κB activation) was significantly higher in tumor cells compared to adjacent and normal epithelial cells [(41.3±3.52)% vs (0.38±0.22) %, t = 10.993, P = 0.000<0.05; (41.3±3.52)% vs (0±0.31)%, t = 11.484, P = 0.000<0.05]. NF-κB activation was correlated with tumor invasion-related clinicopathological features such as lymphatic invasion, pathological stage, and depth of invasion (Z = 2.148, P = 0.032<0.05; χ2 = 8.758, P = 0.033<0.05; χ2 = 18.531, P = 0.006<0.05). NF-κB activation was significantly correlated with expression of heparanase gene (r = 0.194, P = 0.046<0.05).
CONCLUSION: NF-κB RelA (p65) activation was related with increased heparanase gene expression and correlated with poor clinicopathological characteristics in gastric cancers. This suggests NF-κB as a major controller of the metastatic phenotype through its reciprocal regulation of some metastasis-related genes.
- Citation: Cao HJ, Fang Y, Zhang X, Chen WJ, Zhou WP, Wang H, Wang LB, Wu JM. Tumor metastasis and the reciprocal regulation of heparanase gene expression by nuclear factor kappa B in human gastric carcinoma tissue. World J Gastroenterol 2005; 11(6): 903-907
- URL: https://www.wjgnet.com/1007-9327/full/v11/i6/903.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i6.903
Gastric cancer is one of the most common malignant neoplasms in China and its incidence is gradually increasing in recent years. The clinical outcome for gastric cancer patients is still very poor with a 5-year survival rate of only 20% in all stages[1] and only 40% of the patients respond to surgical intervention[2].
NF-κB is involved in the regulation of apoptosis, tumor progression, and responses to chemotherapy and ionizing radiation[3]. But its role in the process of gastric cancer metastasis has not been examined in detail. NF-κB is a transcriptional activator of MMP-9 and uPA[4], supports a role of the NF-κB signal transduction pathway in the metastatic process. However, little information is available concerning the activity of NF-κB in gastric carcinoma, one of the most aggressive types of cancer.
Degradation of basement membrane and extracellular matrix structures are important features of invasion and metastasis in gastric cancer[5]. Human heparanase, an endoglycosidase, specifically involved in cleaving heparan sulfate, has been cloned recently, and it has been reported that this gene is functionally related to the invasion and metastasis of cancer cells[6]. Based on previous reports, we speculate that heparanase may be involved in the invasion and metastasis of gastric cancer cells. In the present study, we examined heparanase expression of the gastric cancer patients using quantitative RT-PCR.
It is recently reported that heparanase expression is regulated by NF-κB in tumor cell lines[7], suggesting an important role for the NF-κB signal transduction pathway in the metastatic process. However, little information is available concerning NF-κB activation and whether it regulates the expression of heparanase expression in gastric carcinoma.
In the present study, we aimed to investigate whether NF-κB was constitutively activated in gastric carcinoma tissues. We also analyzed the correlation between NF-κB activity and clinicopathological features and whether heparanase expression was regulated by NF-κB activation in gastric carcinoma.
Forty-five gastric carcinoma patients who gave informed consent before surgical treatment were enrolled into the present study at Sir Run Run Shaw Hospital between 2000 and 2004. All the normal, adjacent and tumor specimens, including 3 cases of metastatic liver tissues, were obtained from surgically resected tissues in the operating room, and stored in liquid nitrogen until use. The nuclear proteins were extracted immediately. Tissues were fixed in 10% formalin and embedded in paraffin for H&E staining and immunohistochemical staining. All specimens were classified histologically. The clinical pathological characteristics were evaluated according to the guidelines of the Union Internationale Contre le Cancer.
Immunostaining was performed as described previously with a moderate modification[8]. Briefly, antigen retrieval on paraffin sections was performed by heating three times in a 10 mmol/L citrate buffer solution (pH 6.0) in a microwave. Slides were then probed with primary (anti-RelA p65; 1:200; sc-109; Santa Cruz Biotechnology, Santa Cruz, CA), and incubated with secondary antibodies (RelA, goat anti-rabbit immunoglobulin). Antibody binding was detected with a combination of DAB (40 mg/150 mL in PBS; Sigma Biotechnology, USA) and 0.06% hydrogen peroxide (H2O2). The number of nuclear-positive cells was counted and found to be 200 cells for each section. Nuclear staining, which indicated nuclear translocation of RelA, was considered as the marker of NF-κB activation.
Normal, adjacent and tumor specimens were collected in the operating room and homogenized in hypotonic buffer [10 mmol/L HEPES (pH 7.9), 10 mmol/L KCl, 1.5 mmol/L MgCl2, 0.1% NP40, and 5% protease inhibitor (0.2 mmol/L DTT, 10 mmol/L benzamidine, 7 ng/L leupeptin, 50 ng/L soybean trypsin inhibitor, 2 ng/L aprotinin, 2 ng/L antipain, 0.7 ng/L pepstatin, 0.5 mmol/L phenylmethylsulfonyl fluoride, and 0.5 mmol/L 4-(2-aminoethyl) benzenesulfonyl fluoride)] immediately. Homogenized tissues were incubated on ice for 10 min and extraction of nuclear contents was performed as described previously[9]. The protein concentrations were determined by Bradford assay (Bio-Rad, Hercules, CA.). The nuclear extracts were stored at -80 °C until use.
To further prove the results of immunostaining, NF-κB nuclear translocation was analyzed by EMSA for nuclear protein extracts of carcinomas, adjacent and normal tissues as described previously[9]. Nuclear protein extracts (10 μg in each assay) were incubated with the binding buffer [60 mmol/L HEPES (pH 7.5), 180 mmol/L KCl, 15 mmol/L MgCl2, 0.6 mmol/L EDTA, and 24% glycerol], poly (dI-dC) (Amersham Pharmacia Biotech AB, Uppsala, Sweden) and 32P-labeled double-stranded oligonucleotides containing the binding motif of NF-κB (Promega Corp., Madison, WI) for 30 min at 37 °C. The sequence of the double-stranded oligomer used for EMSA was 5’-AGTTGAGGGGACTTTCCCAGGC-3’.
Total RNA was extracted from carcinoma, adjacent and normal tissues by the TRIzol reagent. The primer sequences for amplification of heparanase gene were 5’ AGACCTTTGGGACCTCATGGA 3’ (forward) and 5’ GCAACTTTGGCATTTCTTATCACAA 3’ (reverse), and the probe was 5’ FAM- CAGGAAGTTCACTGGGCTTGCCAGCTTTCTCA -TRAM 3’. The primer sequences for amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene were 5’ CTTAGCACCCCTCCCCAAG 3’ (forward) and 5’ GATGTTCTGGAGAGCCCCG 3’ (reverse) and the probe was 5’ FAM- CATGCCATCACTGCCACCCAGAAGA -TRAM 3’. Expected RT-PCR product sizes were 179 bp for heparanase gene and 150 bp for GAPDH. PCR conditions consisted an initial denaturation step for 5 min at 94 °C, followed by 40 cycles of amplification (denaturation for 30 s at 94 °C, annealing for 15 s at 62 °C, and a final extension for 10 min at 72 °C). The RT-PCR products were sequenced in Tumor Research Institute of Zhejiang University, and the sequences we obtained coincided with the sequences available in GenBank [accession number BC083511 (for GAPDH) and accession number BC051321 (for heparanase)]. The RT-PCR products were also visualized on 2.0% agarose gels stained by ethidium bromide. Based on the fact that the CT (threshold cycle) value was inversely proportional to the log value of the original copy number of the target sequence, heparanase CT/ GAPDH CT was used to evaluate the mRNA expression level in different tissues.
All statistical analyses were performed with SPSS Statistical Software (release 12.0) on the windows workstation. The difference of the mean staining rate among tumor, adjacent and normal cells was analyzed by t-test for paired data. The correlation between the RelA nuclear staining rate and the clinical parameters was analyzed by Rank Sum test. The correlation between RelA nuclear staining and heparanase gene expression was analyzed by Spearman’s test (one-sided). P value <0.05 was considered as significant.
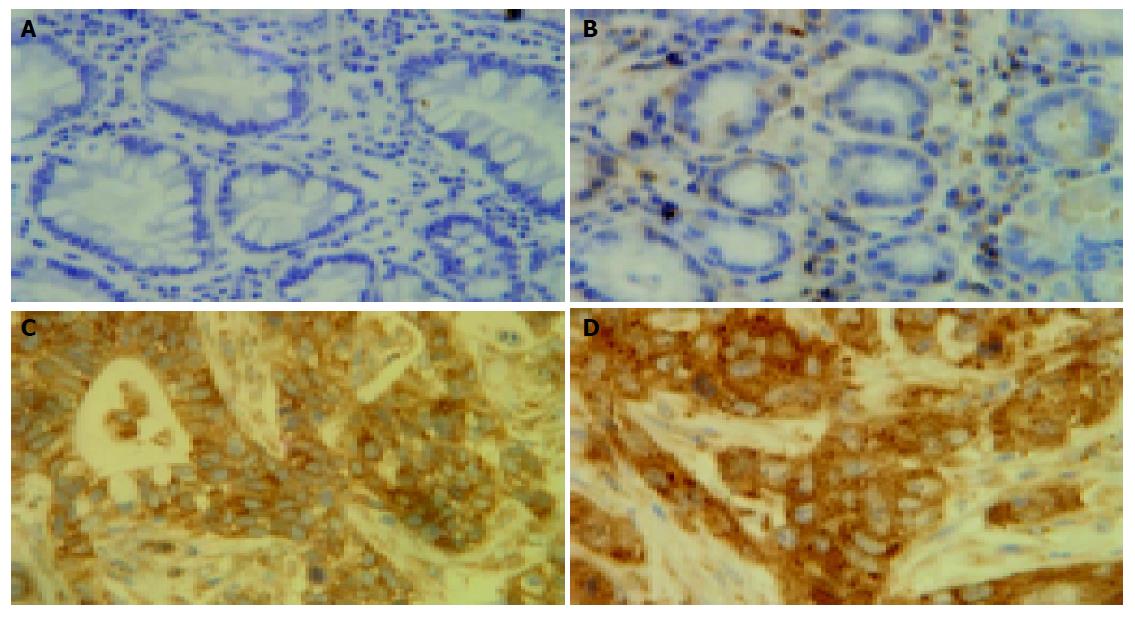
To ascertain whether NF-κB was activated in gastric carcinoma specimens, an immunohistochemical analysis of RelA was performed in formalin-fixed, paraffin-embedded gastric carcinoma specimens (Figure 1). RelA (P65) staining was significantly enhanced in the nuclei of the tumor cells compared to adjacent and normal epithelial cells. RelA staining was markedly higher in the metastatic tissues of the liver in the 3 cases compared to the gastric tumor specimens. The percentages of RelA (P65) staining were (41.3±3.52)%, (0.38±0.22)%, and (0±0.31)% in tumor, adjacent and normal epithelial cells, respectively. The nuclear staining rates were statistically higher in tumor cells compared with those in adjacent and normal epithelial cells (t = 10.993, P = 0.000; t = 11.484, P = 0.000).
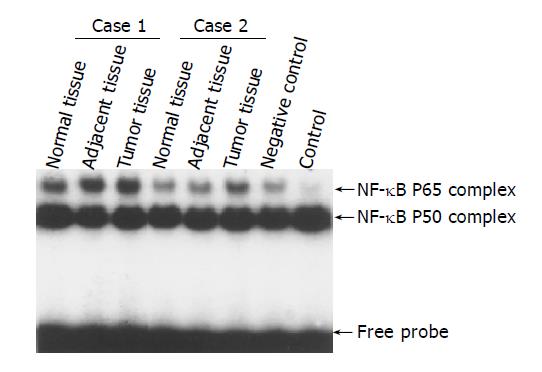
EMSA was used to confirm the increased nuclear translocation of RelA (p65) in gastric carcinoma tissues. Figure 2 showed significantly increased NF-κB RelA (p65) DNA binding activity in tumor tissue compared to adjacent and normal tissues of 2 typical cases.
The correlations between RelA nuclear staining and the clinical pathological parameters in the 45 gastric carcinoma specimens were analyzed statistically as described in “Materials and Methods”. As shown in Table 1, NF-κB activation was correlated with lymphatic invasion (Z = 2.148, P = 0.032), pathological stage (χ2 = 8.758, P = 0.033), and depth of invasion (χ2 = 18.531, P = 0.006).
| Clinical pathological indices | Cases | Mean rank | Value | Asymp. sig. (two-sided) | Test |
| Depth of invasion | 45 | χ2 = 18.531 | 0.006a | Kruskal-Wallis H | |
| Mucosa | 4 | 52.83 | |||
| Submucosa | 2 | 14.5 | |||
| Superficial muscular | 3 | 19.5 | |||
| Deep muscular | 2 | 5.5 | |||
| Serosa | 30 | 32.5 | |||
| Out of serosa | 4 | 5.83 | |||
| Lymphatic permeation | 45 | Z = 2.148 | 0.032a | Mann-Whitney U | |
| Have | 3 | 5.5 0 | |||
| No | 42 | 33.37 | |||
| Stage | χ2 = 8.758 | 0.033 a | Median test | ||
| I | 5 | 35 | |||
| II | 5 | 39.5 | |||
| III | 20 | 30.9 | |||
| IV | 15 | 34.4 | |||
| Histological grading | 45 | χ2 = 8.920 | 0.014 a | Kruskal-Wallis H | |
| Well | 8 | 18.3 | |||
| Middle | 15 | 31.6 | |||
| Poor | 22 | 37.21 |
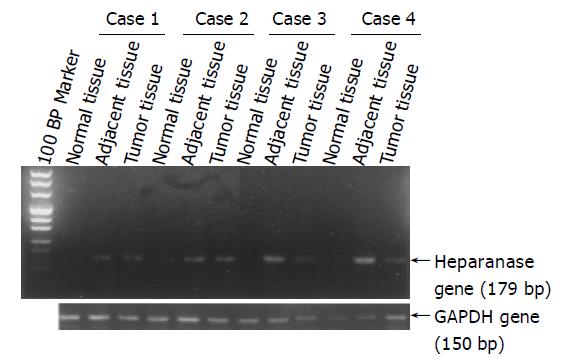
We examined the expression of heparanase in gastric carcinoma specimens by RT-PCR (Figure 3) and quantitative RT-PCR, since the expression of heparanase was thought to be mediated by NF-κB. Heparanase mRNA levels in gastric cancer tissues were significantly higher than those in adjacent and normal tissues (data not included in this paper and will be published in another paper). Correlation analysis between NF-κB activation and heparanase expression with one-sided Spearman’s test showed positive relation (r = 0.194, P = 0.046).
Previously, our studies have shown the activation of NF-κB in various cultured cell lines and several carcinoma tissues. NF-κB activation was usually evaluated on the basis of nuclear translocation of RelA (p65) and/or NF-κB 1 (p50) using EMSA and/or immunohistochemical analysis[10]. In this study, NF-κB RelA (p65) was constitutively activated in gastric carcinoma tissues by immunohistochemical analysis through quantified staining of nuclear RelA (p65) to evaluate NF-κB activation (Figure 1). The results were further proved by EMSAs in several cases. The significance of NF-κB activation in carcinoma tissues remained unclear until now. Here, we analyzed the relationship between NF-κB activation, as estimated by nuclear translocation of RelA, and the clinicopathological parameters. Our data proved that NF-κB activation was correlated with clinicopathological parameters such as lymphatic invasion, pathological stage, and depth of invasion (P = 0.032, P = 0.033 and P = 0.006, separately) in gastric carcinoma tissues(Table 1). These results supported the correlation between NF-κB activation and aggressiveness of gastric carcinomas, which is consistent to reports from other investigators[11,12]. Recent studies also showed that NF-κB is constitutively activated in several other tumors such as pancreatic cancer and breast cancer[13,14]. Interestingly, RelA (p65) staining was especially enhanced in the metastatic specimens of the liver, suggesting that NF-κB activation might contribute to the metastasis to distant organs. Since only 3 cases of liver metastatic specimens were collected in this study, additional specimens would need to be collected for future studies.
Specific enzymes produced and activated by cancer cells contributed to the degradation of extracellular matrices and basement membranes. Overexpression of heparanase was thought to contribute to tumor invasion, metastasis[15], and inflammatory reactions[16], and the enzyme was moderately up-regulated especially in metastatic cancers[17].
In our previous study, the heparanase mRNA levels in gastric cancer specimens were significantly higher than those in adjacent and normal tissues, while there was no significant difference between the adjacent and normal tissues. The heparanase mRNA levels in gastric cancer tissues were significantly correlated with age, invasive depth, differentiation status, tumor size, metastasis, blood vessel invasion, lymphatic vessel invasion and nerves invasion (data were not shown in this paper and will be published in another paper). However, the mechanism leading to the overexpression of heparanase gene in cancer cells remains unclear.
Recently, it was reported that heparanase expression was regulated by NF-κB in tumor cell lines[7]. NF-κB modulated the expression of extracellular matrix proteinases such as MMPs, and the blockage of the NF-κB signal pathway resulted in the down-regulation of MMP-9 and heparanase. However, there is little information on the relationship between the NF-κB activity and the expression of heparanase gene in gastric cancer tissue.
To explore this relationship, the correlation between NF-κB activation and the heparanase gene expression of the invasion-related factor in tumor cells was analyzed by immunohistochemistry and quantitative RT-PCR separately. The results showed that there is a positive correlation between NF-κB activation and the heparanase expression in gastric carcinoma. However, specimens, showing a high heparanse expression, were not always detected corresponding to high NF-κB activation. Thus, this mechanism needs further study. We planned to block the NF-κB/IκB signal transduction pathway in a gastric cancer cell line using either mutant IκB or siRNA of IκB, to study whether the expression of heparanase gene could be down-regulated or not, and to determine how it affects the metastatic activity of tumor cells.
In conclusion, we found that the level of nuclear translocation of NF-κB was higher in gastric carcinoma cells than in adjacent and normal epithelial cells. There is a significant correlation between NF-κB RelA (p65) activation and the expression of heparanase gene. In the future, this positive relationship will be further studied to learn whether blocking the NF-κB signal transduction pathway can inhibit heparanase expression in gastric cancer cell lines. NF-κB and/or heparanase could be potential targets for anti-invasion therapies of gastric carcinoma.
Assistant Editor Li WZ Edited by Gabbe M
| 1. | Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, Vernon SW, Cronin K, Edwards BK. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Ajani JA, Mansfield PF, Ota DM. Potentially resectable gastric carcinoma: current approaches to staging and preoperative therapy. World J Surg. 1995;19:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Bentires-Alj M, Hellin AC, Ameyar M, Chouaib S, Merville MP, Bours V. Stable inhibition of nuclear factor kappaB in cancer cells does not increase sensitivity to cytotoxic drugs. Cancer Res. 1999;59:811-815. [PubMed] |
| 4. | Newton TR, Patel NM, Bhat-Nakshatri P, Stauss CR, Goulet RJ, Nakshatri H. Negative regulation of transactivation function but not DNA binding of NF-kappaB and AP-1 by IkappaBbeta1 in breast cancer cells. J Biol Chem. 1999;274:18827-18835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Tahara E. Molecular biology of gastric cancer. World J Surg. 1995;19:484-488; discussion 489-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Hulett MD, Freeman C, Hamdorf BJ, Baker RT, Harris MJ, Parish CR. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat Med. 1999;5:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 399] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Andela VB, Schwarz EM, Puzas JE, O'Keefe RJ, Rosier RN. Tumor metastasis and the reciprocal regulation of prometastatic and antimetastatic factors by nuclear factor kappaB. Cancer Res. 2000;60:6557-6562. [PubMed] |
| 8. | Zabel U, Henkel T, Silva MS, Baeuerle PA. Nuclear uptake control of NF-kappa B by MAD-3, an I kappa B protein present in the nucleus. EMBO J. 1993;12:201-211. [PubMed] |
| 9. | Huang Y, Fang Y, Wu J, Dziadyk JM, Zhu X, Sui M, Fan W. Regulation of Vinca alkaloid-induced apoptosis by NF-kappaB/IkappaB pathway in human tumor cells. Mol Cancer Ther. 2004;3:271-277. [PubMed] |
| 10. | Huang Y, Fang Y, Dziadyk JM, Norris JS, Fan W. The possible correlation between activation of NF-kappaB/IkappaB pathway and the susceptibility of tumor cells to paclitaxel-induced apoptosis. Oncol Res. 2002;13:113-122. [PubMed] |
| 11. | Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136-4142. [PubMed] |
| 12. | Wang W, Luo HS, Yu BP. Expression of NF-kappaB and human telomerase reverse transcriptase in gastric cancer and precancerous lesions. World J Gastroenterol. 2004;10:177-181. [PubMed] |
| 13. | Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119-127. [PubMed] |
| 14. | Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 542] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99-108. [PubMed] |
| 16. | Mollinedo F, Nakajima M, Llorens A, Barbosa E, Callejo S, Gajate C, Fabra A. Major co-localization of the extracellular-matrix degradative enzymes heparanase and gelatinase in tertiary granules of human neutrophils. Biochem J. 1997;327:917-923. [PubMed] |
| 17. | Marchetti D, Li J, Shen R. Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res. 2000;60:4767-4770. [PubMed] |