Published online Feb 14, 2005. doi: 10.3748/wjg.v11.i6.885
Revised: April 17, 2004
Accepted: May 13, 2004
Published online: February 14, 2005
AIM: To determine the changes of gene expression profile in small intestinal allografts in rats after cold preservation/reperfusion, and to identify the genes relevant to cold preservation/reperfusion injury.
METHODS: Heterotopic segmental small bowel transpla-ntation was performed in six rats with a sham operation and they were used as controls. Total RNA was extracted from the allografts (experimental group) and normal intestines (control group) 1 h after cold preservation/reperfusion, and then purified to mRNA, which was then reversely transcribed to cDNA, and labeled with fluorescent Cy5-dUTP and Cy3-dUTP to prepare hybridization probes. The mixed probes were hybridized to the cDNA microarray. After high-stringent washing, the fluorescent signals on cDNA microarray chip were scanned and analyzed.
RESULTS: Among the 4 096 target genes, 82 differentially expressed genes were identified between the two groups. There were 18 novel genes, 33 expression sequence tags, and 31 previously reported genes. The selected genes may be divided into four classes: genes modulating cellular adhesion, genes regulating cellular energy, glucose and protein metabolism, early response genes and other genes.
CONCLUSION: A total of 82 genes that may be relevant to cold preservation/reperfusion injury in small intestinal allografts are identified. Abnormal adhesion between polymorphonuclears and endothelia and failure in energy, glucose and protein metabolism of the grafts may contribute to preservation/reperfusion injury. The functions of the novel genes identified in our study need to be clarified further.
- Citation: Wang SF, Liang Q, Li GW, Gao K. Gene expression profile in rat small intestinal allografts after cold preservation/reperfusion. World J Gastroenterol 2005; 11(6): 885-889
- URL: https://www.wjgnet.com/1007-9327/full/v11/i6/885.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i6.885
Primary dysfunction of small intestinal grafts induced by rejection and cold preservation/reperfusion injury, is still one of the main obstacles in clinical small intestinal transplantation[1-6]. The cold preservation/reperfusion injury of small intestinal grafts, an unspecific injury, may enhance the immunogenicity of the grafts, and exacerbate the degree of acute or chronic rejection, thus influence the survival and function of the grafts[7]. Therefore, better understanding of molecular mechanism in the course of cold preservation/reperfusion is beneficial for searching for new methods that could decrease the graft injury. However, previous studies only investigated the relationship between one or a few genes or proteins and cold preservation/reperfusion injury by Northern hybridization or RT-PCR. Thus, the overall changes in intra-graft gene expression profile are not clearly identified. The aim of this study was to determine the gene expression profile of rat small bowel allograft after cold preservation/reperfusion injury, and to identify the genes relevant to cold preservation/reperfusion injury by means of cDNA microarray.
Healthy male Sprague-Dawley (SD) rats, weighing 280±20 g, provided by Medical Experiment Animal Center, Xi’an Jiaotong University, were housed in standard animal facilities, fed with commercially available rat chow and tap water ad libitum for 1 wk before test to acclimatize the laboratory, and maintained on a 12-h light/dark cycle.
SD rats were used as donors and recipients. They were randomly divided into two groups: sham operation group (S group, n = 6), in which animals were only subjected to anesthesia and laparotomy and left nephrectomy, and heterotopic segmental small bowel transplantation group (SBT group, n = 12), in which the donor and recipient were paired according to the similar body weight.
Heterotopic segmental small bowel transplantation was performed with a technique modified from that described by Monchik and others[8]. All animals were fasted for 12 h, but had free access to water before operation. They were anesthetized with pentobarbital sodium solution (2%, 35 mg/kg) intraperitoneally. After perfused in situ with cold lactated Ringer’s solution, the small intestine 5 cm distal to Treitz ligament was harvested from each donor rat with a vascular pedicle including portal vein (PV) and aorta segment with superior mesenteric artery, and stored at 4 °C in lactated Ringer’s solution for 1 h. After left nephrectomy, the left renal vein and abdominal aorta of the recipient rat were isolated. The graft was revascularized by end-to-side anastomosis between donor aorta and recipient aorta using the continuous 9-0 non-traumatic nylon sutures and end-to-side anastomosis between donor PV and left renal vein of the recipient using a cuff technique. The recipient’s own intestine was left intact. During transplantation, mean warm ischemia time of graft was less than 1 min, and cold preservation time of graft did not exceed 60 min.
Four thousand and ninety-six target cDNA clones were used in the cDNA chip (provided by Biostar Gene Ltd, Shanghai, China). These genes were amplified through polymerase chain reaction (PCR) using universal primers and purified with a standard method. The quality of PCR was monitored by agarose electrophoresis. Target genes were dissolved in 3×standard saline citrate (SSC) spotting solution, and spotted on silylated slides (Telechem, Inc., USA) by Cartesian 7500 spotting Robotics (Cartesian, Inc., USA). After spotting, the slides were hydrated for 2 h, dried for 0.5 h at room temperature, cross-linked under UV light and then treated with 0.2% sodium dodecyl sulfate (SDS), H2O and 0.2% NaBH4 for 10 min, respectively. The slides were then dried in the cold and ready for use.
In SBT group, the small intestinal grafts were cut 3 cm long and kept in liquid nitrogen immediately after successful revascularization and 1 h after reperfusion of the small intestinal grafts. Samples taken from the same anatomic sites in the S group were kept in liquid nitrogen. Total RNA in different groups was extracted by a single step method. UV light analysis and electrophoresis detection showed the good quality of purified RNA. mRNA was isolated and purified using an oligotex mRNA Midi kit (Qiagen, Inc., California, USA). The fluorescein-labeled cDNA probes were prepared through retro-transcription and purified according to the method of Schena et al[9]. Probes from normal small intestine in S group were labeled with Cy3-dUTP, and those from small intestinal graft in SBT group with Cy5-dUTP. The probes were mixed, precipitated by ethanol and resolved in 20 μL hybridization solution (5×SSC+0.2% SDS).
Hybridization probes and gene chip were denatured in a 95 °C bath for 5 min. The probes were added on the chip and covered with a glass. The chip was hybridized in a sealed chamber at 60 °C for 15-17 h. After removing the cover glass, the chip was washed in 2×SSC+0.2%SDS, 0.1×SSC+ 0.2%SDS and 0.1×SSC, 10 min each, respectively, and then dried at room temperature.
The chip was scanned with a Scan Array 4000 scanner (General scanning Inc., USA) at two wavelengths to detect emission from both Cy3 and Cy5. The acquired images were analyzed using ImaGene 3.0 software (BioDiscovery, USA). The fluorescent intensity of each spot at the two wavelengths represented the quantity of Cy3-dUTP and Cy5-dUTP, respectively, hybridized to each spot. Cy3 and Cy5 overall intensity was normalized and corrected by a coefficient according to location ratios of the housekeeping genes. The criteria for screening out each differentially expressed gene were defined: (1) The absolute value of the natural logarithm of the signal ratio of Cy5/Cy3 was greater than 0.69; or the ratio of Cy5/Cy3>2.0 or <0.5, or (2) one of the signal values of Cy3 and Cy5 was greater than 800.
The microarray consisted of 4096 sequences of rats including full length and partial complementary DNA (cDNA). Its standards of quality control included 20 housekeeping genes as positive controls, 16 plant genes as negative controls and, 19 spotting solutions as blank controls. The results of this study completely accorded with the above standards of quality control, which indicated that there was no contamination during the process of study.
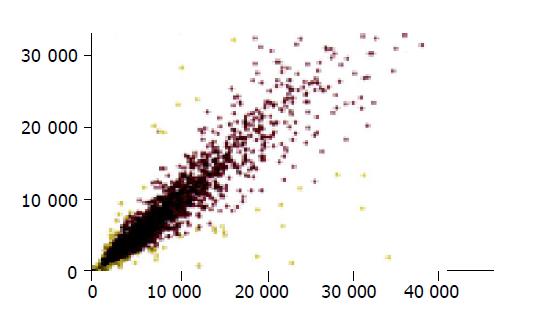
The scatter plot, derived from Cy3 and Cy5 fluorescent signal values, revealed a dispersed distribution pattern (Figure 1). Every spot represented a gene. Most spots were found on an almost 45o diagonal line. Certain yellow spots were distributed beyond the 45o diagonal line, which indicated the existence of some differentially expressed genes between normal small intestine and small intestinal graft. The genes with a remarkably different expression distributed on the upper and lower sides of the 45o diagonal line, representing the up-regulation and down-regulation, respectively. Red spots showed signal differences between 0.5 and 2 folds, which indicated the non-differentially expressed genes close to the 45o diagonal line.
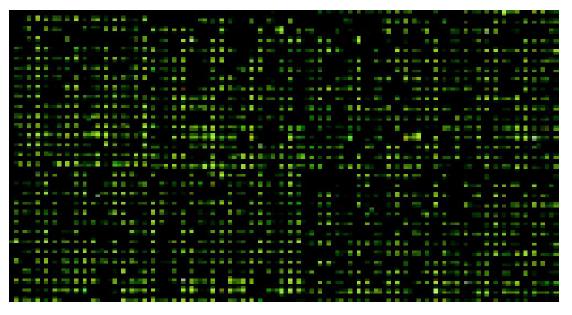
The fluorescent scanning profile of gene expression was merged by computer to produce the Figure 2. Yellow spots indicated no significant difference in gene expression between normal small intestine (Cy3) and that of small intestinal graft (Cy5). Green spots indicate that gene expression was higher in normal small intestine than in small intestinal grafts, whereas red spots indicate the opposite.
Overall, 82 differentially expressed genes between normal small intestines and small intestinal grafts were detected. Among them, 31 genes with GenBank identity numbers included 17 down-regulation genes and 14 up-regulation genes (Table 1). Expression sequence tags (EST) counted to 33. In addition, there were 18 novel genes, eight up-regulated genes and 10 down-regulated genes. The functions of these novel genes need to be investigated further.
| GenBank ID | Definition | Ratio Cy5/Cy3) |
| M 15327 | Rat alcohol dehydrogenase mRNA | 0.05 |
| U 76714 | Cell adhesion regulator mRNA | 0.233 |
| NM-016998 | Carboxypeptidase mRNA | 0.243 |
| NM-024391 | 17-beta hydroxysteroid dehydrogenase mRNA | 0.291 |
| NM-019158 | Aquaporin 8 mRNA | 0.304 |
| NM-017272 | Aldehyde dehydrogenase family mRNA | 0.337 |
| NM-013098 | Glucose-6-phosphatase 1 mRNA | 0.361 |
| NM-031639 | Discs, large homolog 3 mRNA | 0.396 |
| NM-022407 | Aldehyde dehydrogenase subfamily A1 mRNA | 0.406 |
| X05080 | Beta-globin | 0.422 |
| M83143 | Beta-galactoside-alpha2,6-sialyltransferase mRNA | 0.429 |
| AF 154572 | ERG2 protein mRNA | 0.436 |
| NM-031351 | Attractin mRNA | 0.45 |
| NM-013080 | Protein tyrosine phosphatase, receptor-type mRNA | 0.45 |
| NM-031565 | Carboxylesterase 1 mRNA | 0.461 |
| NM-031579 | Protein tyrosine phosphatase 4al mRNA | 0.465 |
| NM-053297 | Pyruvate kinase 3 mRNA | 0.479 |
| NM-054008 | Rgc 32 protein mRNA | 2.053 |
| NM-020538 | Arylacetamide deacetylase mRNA | 2.056 |
| M 20406 | P450 II B mRNA | 2.083 |
| NM-053523 | Herpud 1 mRNA | 2.085 |
| AJ 223184 | DORA Protein | 2.21 |
| NM-022392 | Growth response protein mRNA (CL-6) | 2.489 |
| NM-012771 | Lysozyme mRNA | 2.491 |
| X 68312 | Ig rearranged mu-chain C region | 2.495 |
| NM-017268 | Hmgcsl mRNA | 2.497 |
| NM-053372 | Secretory leukocyte protease inhibitor mRNA | 2.796 |
| AJ 238392 | Sulfotransferase K2 mRNA | 2.876 |
| AF 146518 | Amino peptidase A short variant mRNA | 2.883 |
| NM 017211 | Selectin, endothelial cell ligand mRNA | 2.949 |
| NM-022251 | Aminopeptidase A Mrna | 3.302 |
Small bowel transplantation has been advocated as the preferred therapy for patients with irreversible intestinal failures such as short bowel syndrome[10-14]. However, primary graft rejection and cold preservation/reperfusion damage involved in loss of small intestine function after transplantation are still the major obstacles to small bowel transplantation. At present, in addition to theory of free radical species and theory of calcium overload, the mechanism of cold preservation/reperfusion injury has not yet completely elucidated. In order to modulate this non-specific injury, it is necessary to investigate the molecular and genetic mechanism of cold preservation/reperfusion injury. In this study, we identified some genes that may be relevant to cold preservation/reperfusion damage by use of cDNA microarray. The selected genes could be divided into four classes: genes modulating cellular adhesion, genes regulating cellular energy, glucose and protein metabolism, early response genes and other genes.
In physiological conditions, the repelling action between the vascular endothelia and polymorphonuclears flowing in the blood vessels guarantees the microcirculatory perfusion. Our experiments showed that the expression of cell adhesion regulator genes (U76714) and receptor-typed protein-tyrosine-phosphatase (NM-013080) with function of inhibiting cell adhesion decreased 1 h after cold preservation/reperfusion of grafts, whereas the expression of DORA protein (AJ223184), an adhesion molecule of immunoglobulin superfamily, and selectin family (NM-017211) increased. Thus, these changes may induce the adhesion of polymorphonuclears to the endothelia, and initiate the adhesion cascade reaction. Adhesion of polymorphonuclears to the endothelia and the recruitment of the cells into the grafts are essential for the development of cold preservation/reperfusion injury. In the process, selectins play an important role[15,16]. The results of cDNA microarray showed that 1 h after cold preservation/reperfusion selectins revealed up-regulation, which directed polymorphonuclears accumulation in the grafts and activated polymorphonuclears to release inflammatory mediators and proteases. Injury to the graft may occur through the following ways. Firstly, adhesion of polymorphonuclears to the endothelia causes the damage and physical obstruction of capillaries, such as the no reflow phenomenon, which injures the graft further. Secondly, a great amount of free radical species is produced through the respiratory burst of polymorphonuclears, which destroy the structure of membrane. Thirdly, polymorphonuclears secrete proteolytic enzymes such as elastase that could decompose the graft. Therefore, anti-adhesion therapy is likely to be a new approach to relieve/inhibit cold preservation/reperfusion injury of small intestinal transplant.
Our study showed that the expression of cytochrome P450 (M20406) of respiratory chain of biological oxidation increased in the course of cold preservation/reperfusion of small intestinal grafts, whereas that of attractin (NM-031351), which controls homeostasis of cell energy metabolisms, decreased, suggesting that there is a failure in energy and substance metabolism of cells[17,18]. The injury of capillaries mediated by adhesion cascade reaction made the grafts in the state of relative anoxia during reperfusion since the biological oxidation of glucose mainly depended on glycolysis. In our study, pyruvate-kinase (NM-053297), the key enzyme in the course of glycolysis, expressed downward, which hindered glycolysis. In the meantime, the expression of glucose-6- phosphatase (NM-013098), the key enzyme in the course of glyconeogenesis, was also down regulated, which inhibited glyconeogenesis. Although the expression of aminopeptidase A (AF146518, NM-022251), which hydrolyzes the peptide chains, increased, the expression of carboxypolypeptidase A (NM-016998), which hydrolyzes the peptide bonds and releases amino acids, expressed downward, resulting in the failure of peptide or protein utilization by the cells. In addition, due to the action of the oxygen-free radical, peroxide lipid produced in the course of reperfusion of small intestinal grafts usually degrades, and forms some aldehyde compounds like malonic aldehyde, which easily causes the occurrence of additional response with cell proteins, leading to the cross-linking of intra-molecules or inter-molecules of proteins and subsequently resulting in coagulation. In our study, aldehyde dehydrogenases (NM-022407 and NM-017272), which oxidize and decompose the aldehyde, expressed downward, which may and aggravate the harm to the cells[19,20]. Therefore, there is not only the disturbance of biological oxidation of glucose or proteins but also the failure of catabolism of toxic products, such as the aldehyde during the reperfusion of small intestinal grafts.
Early response genes of cells, a kind of important mediated genes, determine the final response mode of cells stimulated in the external environment, namely expression of the function-relevant gene downstream[21]. Our study showed that early response genes of cells, growth response protein (CL-6 or NM-022392) and Rgc32 protein (NM-054008) expressed upward whereas protein tyrosine phosphatase (PTPase or NM-031579) expressed downward during cold preservation/reperfusion of small intestinal grafts. Rgc32 protein increases the activation of CDC2 kinase that cell cycle depends on, and impels the synthesis of cellular DNA to enter the S phase, indicating a function of activating cell cycle. CL-6 also plays an important role in cell proliferation. Therefore, the cold preservation/reperfusion of intestinal grafts triggers cell proliferation of small intestine and promotes the recovery of injury. This conclusion coincides with those obtained from previous studies at the single gene level[22].
Our study also indicates that endogenous protection in small intestinal grafts is limitedly initiated through adjustment of the expression mode of genes, which may relieve the reperfusion injury. This finding has seldom been observed in previous studies. Aquaporin 8 (NM-019158) expressed downward, which could lessen cell edema caused by relative lack of oxygen[23]. Secretory protease inhibitor of leukocyte (NM-022407) expressed upward, which could suppress protease released by adhesion cascade reaction activated by endothelia and polymorphonuclears, and lessen the hydrolysis of proteins[24].
In conclusion, our results using cDNA microarray are consistent with previous studies at the single gene level. Moreover, there are changes in the gene expression mode after the cold preservation/reperfusion of small intestinal grafts on more concrete links, which enable us to further understand the molecular mechanisms of the cold preservation/reperfusion injury. Furthermore, the functions of many new genes identified in our study in the cold preservation/reperfusion injury of small intestinal grafts need to be clarified further.
Edited by Xia HHX Proofread by Chen WW
| 1. | Mittal NK, Tzakis AG, Kato T, Thompson JF. Current status of small bowel transplantation in children: update 2003. Pediatr Clin North Am. 2003;50:1419-133, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Abu-Elmagd K, Bond G. Gut failure and abdominal visceral transplantation. Proc Nutr Soc. 2003;62:727-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Johnson S, Qi S, Xu D, Jolicoeur M, Liu D, Barama A, Busque S, Smeesters C, Daloze P, Chen H. Synergistic effects of RAD and Neoral in inhibition of host-vs.-graft and graft-vs.-host immune responses in rat small-bowel transplantation. Microsurgery. 2003;23:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Farmer DG, Amersi F, Shen XD, Gao F, Anselmo D, Ma J, Dry S, McDiarmid SV, Shaw G, Busuttil RW. Improved survival through the reduction of ischemia-reperfusion injury after rat intestinal transplantation using selective P-selectin blockade with P-selectin glycoprotein ligand-Ig. Transplant Proc. 2002;34:985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Guo WH, Chan KL, Fung PP, Chan KW, Tam PK. Nitric oxide protects segmental intestinal grafts from ischemia and reperfusion injury. Transplant Proc. 2000;32:1297-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Carey HV, Mangino MJ, Southard JH. Changes in gut function during hibernation: implications for bowel transplantation and surgery. Gut. 2001;49:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Massberg S, Messmer K. The nature of ischemia/reperfusion injury. Transplant Proc. 1998;30:4217-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery. 1971;70:693-702. [PubMed] |
| 9. | Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci USA. 1996;93:10614-10619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 958] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 10. | Kato T, Ruiz P, Thompson JF, Eskind LB, Weppler D, Khan FA, Pinna AD, Nery JR, Tzakis AG. Intestinal and multivisceral transplantation. World J Surg. 2002;26:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Park BK. Intestinal transplantation in pediatric patients. Prog Transplant. 2002;12:97-113; quiz 114-115. [PubMed] |
| 12. | Kaufman SS. Small bowel transplantation: selection criteria, operative techniques, advances in specific immunosuppression, prognosis. Curr Opin Pediatr. 2001;13:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Dionigi P, Alessiani M, Ferrazi A. Irreversible intestinal failure, nutrition support, and small bowel transplantation. Nutrition. 2001;17:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Reyes J. Intestinal transplantation for children with short bowel syndrome. Semin Pediatr Surg. 2001;10:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Cooper D, Chitman KD, Williams MC, Granger DN. Time-dependent platelet-vessel wall interactions induced by intestinal ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1027-G1033. [PubMed] |
| 16. | Xia G, Martin AE, Besner GE. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kuramoto T, Kitada K, Inui T, Sasaki Y, Ito K, Hase T, Kawagachi S, Ogawa Y, Nakao K, Barsh GS. Attractin/mahogany/zitter plays a critical role in myelination of the central nervous system. Proc Natl Acad Sci USA. 2001;98:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | He L, Gunn TM, Bouley DM, Lu XY, Watson SJ, Schlossman SF, Duke-Cohan JS, Barsh GS. A biochemical function for attractin in agouti-induced pigmentation and obesity. Nat Genet. 2001;27:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Wang RS, Nakajima T, Honma T. Trichloroethylene inhibits aldehyde dehydrogenase only for aliphatic aldehydes of short chains in rats. Toxicology. 1999;132:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Hsu LC, Chang WC, Hoffmann I, Duester G. Molecular analysis of two closely related mouse aldehyde dehydrogenase genes: identification of a role for Aldh1, but not Aldh-pb, in the biosynthesis of retinoic acid. Biochem J. 1999;339:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Itoh H, Yagi M, Fushida S, Tani T, Hashimoto T, Shimizu K, Miwa K. Activation of immediate early gene, c-fos, and c-jun in the rat small intestine after ischemia/reperfusion. Transplantation. 2000;69:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Taguchi T, Shima Y, Nakao M, Fujii Y, Tajiri T, Ogita K, Suita S. Activation of immediate early genes in relation to proliferation and apoptosis of enterocytes after ischemia-reperfusion injury of small intestine. Transplant Proc. 2002;34:983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | García F, Kierbel A, Larocca MC, Gradilone SA, Splinter P, LaRusso NF, Marinelli RA. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. J Biol Chem. 2001;276:12147-12152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Song Xy, Zeng L, Jin W, Thompson J, Mizel DE, Lei K, Billinghurst RC, Poole AR, Wahl SM. Secretory leukocyte protease inhibitor suppresses the inflammation and joint damage of bacterial cell wall-induced arthritis. J Exp Med. 1999;190:535-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |