Published online Feb 14, 2005. doi: 10.3748/wjg.v11.i6.871
Revised: August 21, 2004
Accepted: October 7, 2004
Published online: February 14, 2005
AIM: To evaluate the expression of matrix metalloproteinase-9 (MMP-9) and its clinical significance in esophageal squamous cell carcinoma (ESCC).
METHODS: The expression of MMP-9 in 208 cases of ESCC was detected by immunohistochemistry (IHC) and its clinical significance in ESCC especially the relationship with the clinicopathological parameters was analyzed.
RESULTS: The percentage of positive cases for MMP-9 detected by IHC was 49.0%. MMP-9 was mainly expressed in the cytoplasm of cancer cells especially in the invasive front. Only weak expression was detected in the stromal cells and no expression in non-cancerous mucosa. The expression of MMP-9 was positively correlated with poorer differentiation (P = 0.001<0.01), existence of vessel permeation (P = 0.027<0.05) and lymph node metastasis (P = 0.027<0.05).
CONCLUSION: The expression of MMP-9 correlates with the cancer cell differentiation, vessel permeation and lymph node metastasis. It may be a novel biomarker for the diagnosis and treatment of ESCC.
- Citation: Gu ZD, Chen KN, Li M, Gu J, Li JY. Clinical significance of matrix metalloproteinase-9 expression in esophageal squamous cell carcinoma. World J Gastroenterol 2005; 11(6): 871-874
- URL: https://www.wjgnet.com/1007-9327/full/v11/i6/871.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i6.871
Esophageal squamous cell carcinoma (ESCC) is one of the most aggressive malignant tumors, and China is one of the leading ESCC high-incidence areas in the world. In general, patients with ESCC have a worse prognosis than those with other digestive tract cancers even after curative surgical resection, due to the extensive local invasion and frequent regional lymph node metastasis at presentation. Spread of malignant tumors is a multi-step process and many of the stages of tumor invasion require degradation or breakdown of the extracellular matrix and connective tissue surrounding the tumor cells. The matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases collectively capable of degrading essentially all components of extracellular matrix (ECM)[1-3], and there is considerable evidence to indicate that individual MMPs have an important role in tumor invasion and metastasis[4-7].
At present, more than 20 members of the human MMP gene family are known, and they are classified into subgroups of collagenases, stromelysins and gelatinases based on their structure and substrate specificity. MMP-9 is one of the gelatinases that mainly degrades type IV collagen, which is the main component of basement membrane. Then the activity of MMP-9 is suggested to be associated with the disruption of basement membrane and also to play a very important role in the distant metastasis potential of carcinoma cells through vessel permeation. Recently, some studies have indicated that MMP-9 is associated with the ESCC[8,9]. However, there is still lack of comprehensive analysis for the expression of MMP-9 in ESCC in large sections of examples.
In this study, immunohistochemistry (IHC) was performed to detect the expression of MMP-9 and the rel-ationship of its expression to clinicopathological parameters was analyzed. We found that the expression of MMP-9 correlates with cancer cell differentiation, vessel permeation and lymph node metastasis, and hence might be a novel biomarker for the diagnosis and treatment of ESCC.
Paraffin-embedded tumor specimens from 208 patients with ESCC who were surgically treated in Peking University School of Oncology from July 1996 to November 2002 were used for IHC analysis, 162 males and 46 females, aged 38-78 years (mean 60 years).
The 4-μm sections were dewaxed in xylene, rehydrated in alcohol, and immersed in 3% hydrogen peroxide for 10 min to suppress endogenous peroxidase activity. Then antigen retrieval was performed by microwave treatment (650 W) of the sections for 10 min in 0.01 mol/L sodium citrate buffer (pH 6.0). After being rinsed for 5 min3 times in PBS, the sections were incubated for 18 h at 4 °C with a mouse antihuman MMP-9 antibody (Novocastra Laboratories, 15W2, 1:20) diluted in PBS. After washing for 5 min×3 times in PBS, the sections were incubated with horseradish peroxidase-labeled goat antimouse immunoglobulin (DAKO, K4001) for 1 h at room temperature. After 3 additional washes, peroxidase activity was developed with diaminobenzidine (DAB) at room temperature. Finally, the sections were counterstained in Mayer’s hematoxylin.
Immunostaining signals were scored by two independent observers. The scores were calculated as the number of stained cells divided by the total number of carcinoma cells. Unequivocal staining of the cytoplasm in more than 10% of carcinoma cells was considered positive.
SPSS 10.0 software was used to perform the χ2 test to analyze the relationship between the expression of MMPs and clinicopathological parameters. P value less than 0.05 was considered statistically significant.
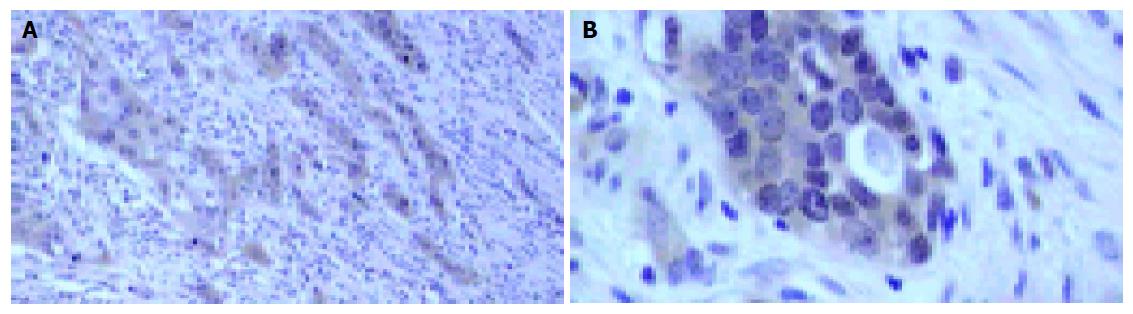
The percentage of positive cases for MMP-9 detected by IHC is 49.0% and Figure 1 shows the representative results. The expression of MMP-9 is mainly located in the cytoplasm of cancer cells. And the immunostaining showed a tendency to be stronger in deeply invading nests especially in invasive fronts. Weak staining was seen in stromal cells and no staining was seen in normal esophageal mucosa.
The expression of MMP-9 was positively correlated with poorer differentiation, existence of vessel permeation and lymph node metastasis, but had no relationship with other parameters (Table 1).
| Clinicopathological parameters | MMP-9 expression | χ2 | P | ||
| Positive cases (%) | Negative cases (%) | ||||
| Age (yr) | <60 | 44 (49.4) | 45 (50.6) | 0.01 | 0.921 |
| >60 | 58 (48.7) | 61 (51.3) | |||
| Sex | Male | 74 (45.7) | 88 (54.3) | 3.308 | 0.069 |
| Female | 28 (60.9) | 18 (39.1) | |||
| Tumor location | Upper | 8 (50.0) | 8 (50.0) | 1.484 | 0.476 |
| Middle | 69 (51.9) | 64 (48.1) | |||
| Lower | 25 (42.4) | 34 (57.6) | |||
| Tumor cell differentiation | Well | 25 (32.5) | 52 (67.5) | 13.503 | 0.001 |
| Middle | 46 (59.7) | 31 (40.3) | |||
| Poor | 31 (57.4) | 23 (42.6) | |||
| Vessel permeation | Absent | 75 (45.2) | 91 (54.8) | 4.896 | 0.027 |
| Present | 27 (64.3) | 15 (35.7) | |||
| Tumor invasion (T) | T1 | 8 (57.1) | 6 (42.9) | 1.305 | 0.521 |
| T2 | 19 (42.2) | 26 (57.8) | |||
| T3 | 75 (50.3) | 74 (49.7) | |||
| Lymph node metastasis (N) | N0 | 45 (41.7) | 63 (58.3) | 4.885 | 0.027 |
| N1 | 57 (57.0) | 43 (43.0) | |||
| TNM stage | I | 7 (58.3) | 5 (41.7) | 7.139 | 0.068 |
| IIa | 38 (39.6) | 58 (60.4) | |||
| IIb | 7 (46.7) | 8 (53.3) | |||
| III | 50 (58.8) | 35 (41.2) | |||
It is widely accepted that cancers develop and progress through the accumulation of various genetic alterations. ESCC is one of the most aggressive carcinomas and the postoperative outcome remains unsatisfactory[10]. But it is recognized that some patients who undergo a curative operation do gain a long-term survival, even though the carcinoma has reached an advanced stage[11]. That is, the widely used TNM staging cannot accurately predict the prognosis in all the patients. So it is important to study some useful biologic markers as indicators for the malignant potential[12]. MMPs have attracted more and more attention because of their important function in tumor invasion, angiogenesis and metastasis. In the present study, we detected the expression of MMP-9 to figure out the role of MMPs in ESCC.
The results of IHC for MMP-9 showed that the immuno-staining was mainly located in the cytoplasm of cancer cells especially the cancer cells at the invasive front. There were weak expressions in stromal cells and no expression in non-cancerous mucosa. This expression status showed that the expression of MMP-9 was elevated in carcinoma tissues compared to non-cancerous mucosa, which indicates that MMP-9 may play a role in the development of ESCC. The high expression of MMP-9 at the invasive front may enforce the degradation of the ECM and then facilitate the invasion or metastasis of tumor cells. Some authors suggest that the expression of MMPs in stromal cells may exert more important function[13,14], but others do not think so[3,4]. In our study, it was found that the expression of MMP-9 was mainly located in the cytoplasm of cancer cells, which indicates that the MMP-9 expressed in cancer cells may play a more important role.
MMP-9 is important in many aspects of biology; ranging from cell proliferation, differentiation and remodeling of the ECM, these events may be the reasons for MMP-9 expression being related to the clinicopathological parameters.
Poor differentiated ESCC has high MMP-9 expression. This may explain why poorly differentiated ESCC has high malignant tendency. High expression of MMP-9 can provide the cancer cell more chances to invade and metastasize.
MMP-9 expression is positively correlated with the existence of vessel permeation (vascular or lymphatic). There may be two reasons for this. One is, MMP-9 mainly degrades type IV collagen, which is the principal component of basement membrane. So the high expression of MMP-9 may facilitate the cancer cells to penetrate the vessel membranes and then to enter the blood stream or metastasize to lymph nodes. Kim et al[15] found that MMP inhibitors can block intravasation of tumor cells and only MMP-9-expressing cells are able to enter the blood stream, which indicates that MMP-9 is required for intravasation and hematogenous spread of tumor cells in vivo. The other reason is that MMP-9 expression can promote the tumor angiogenesis, which can provide oxygen and nutrition for cancer cells, and promote the formation of cancer emboli. Bergers et al[16] suggested that MMP-9 can induce angiogenesis by releasing sequestered VEGF.
High expression of MMP-9 is positively correlated with lymph node metastasis. The mechanism may be the same as that mentioned above. Many authors reported that lymph node metastasis is an independent prognostic factor of ESCC[17,18], and MMP-9 may affect the prognosis by affecting the lymph node metastasis.
On the other hand, the expression of MMP-9 has no relationship with the depth of tumor invasion, which may be due to the fact that the main substrate of MMP-9 is type IV collagen and not type I collagen. Type I collagen is the main component of connective tissue, and the tumor invasion to the deeper layer is always accompanied with the lysis of type I collagen. So, maybe collagenases play an important role in the tumor invasion depth.
In our study, MMP-9 was highly expressed in poor prognostic potential patients, weakly expressed in good prognostic potential patients and did not express in non-cancerous mucosa. This expression pattern lends considerable support to the likelihood that MMP-9 might be acting as a novel biomarker for the diagnosis and treatment of ESCC. Now many potential inhibitors of MMPs, including MMP-9, are assessed for anticancer properties such as synthetic low-molecular weight MMPIs and bryostatin compounds, but there are still some uncertainties and a definite conclusion awaits more studies[19-21].
We thank Dr. Yu Wang, Yun-Tao Xie, Ai-Ping Lu, Yan-Hua Yuan, Feng-ling Wan, Zhen-Yuan Sun and Ye Xu for their directions and excellent technical support.
Assistant Editor Guo SY Edited by Gabbe M
| 1. | Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2922] [Cited by in RCA: 2889] [Article Influence: 120.4] [Reference Citation Analysis (0)] |
| 2. | Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1868] [Cited by in RCA: 1905] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 3. | Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 476] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Murray GI, Duncan ME, O'Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 262] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Talvensaari-Mattila A, Pääkkö P, Höyhtyä M, Blanco-Sequeiros G, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer. 1998;83:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Fong KM, Kida Y, Zimmerman PV, Smith PJ. TIMP1 and adverse prognosis in non-small cell lung cancer. Clin Cancer Res. 1996;2:1369-1372. [PubMed] |
| 7. | Chen JQ, Zhan WH, He YL, Peng JS, Wang JP, Cai SR, Ma JP. Expression of heparanase gene, CD44v6, MMP-7 and nm23 protein and their relationship with the invasion and metastasis of gastric carcinomas. World J Gastroenterol. 2004;10:776-782. [PubMed] |
| 8. | Sier CF, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB, Verspaget HW. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer. 1996;74:413-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 223] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Ohashi K, Nemoto T, Nakamura K, Nemori R. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer. 2000;88:2201-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Chen KN, Xu GW. The diagnosis and treatment for esophageal cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:196-202. |
| 11. | Chen KN, Cheng BC, Shi XT, Ma JS. The diagnosis and treatment for recurrent dysphagia of esophageal carcinoma after radical radiotherapy. Chin J Cancer Res. 1998;10:71-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 12. | Chen KN, Zhang LJ, Shi XT, Cheng BC, Xu GW. The advances in molecular biology for esophageal cancer. Zhongguo Xiongxin Xueguan Waike Linchuang Zazhi. 1999;6:57-60. |
| 13. | Yamashita K, Mori M, Kataoka A, Inoue H, Sugimachi K. The clinical significance of MMP-1 expression in oesophageal carcinoma. Br J Cancer. 2001;84:276-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P. Tumour invasion and matrix metalloproteinases. Crit Rev Oncol Hematol. 2004;49:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Kim J, Yu W, Kovalski K, Ossowski L. Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell. 1998;94:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 338] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2146] [Cited by in RCA: 2040] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 17. | Etoh T, Inoue H, Yoshikawa Y, Barnard GF, Kitano S, Mori M. Increased expression of collagenase-3 (MMP-13) and MT1-MMP in oesophageal cancer is related to cancer aggressiveness. Gut. 2000;47:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Chen KN, Xing HP, Cheng BC, Shi XT, Feng RQ. Expression of mdr-1 gene in cancer tissue and its association with morphological indexes of esophageal carcinoma in Anyang. Chin J Cancer Res. 1997;9:41-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Macaulay VM, O'Byrne KJ, Saunders MP, Braybrooke JP, Long L, Gleeson F, Mason CS, Harris AL, Brown P, Talbot DC. Phase I study of intrapleural batimastat (BB-94), a matrix metalloproteinase inhibitor, in the treatment of malignant pleural effusions. Clin Cancer Res. 1999;5:513-520. [PubMed] |
| 20. | Wylie S, MacDonald IC, Varghese HJ, Schmidt EE, Morris VL, Groom AC, Chambers AF. The matrix metalloproteinase inhibitor batimastat inhibits angiogenesis in liver metastases of B16F1 melanoma cells. Clin Exp Metastasis. 1999;17:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkilä P, Kantor C, Gahmberg CG, Salo T, Konttinen YT. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 403] [Article Influence: 15.5] [Reference Citation Analysis (0)] |