Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.760
Revised: February 4, 2004
Accepted: March 4, 2004
Published online: February 7, 2005
AIM: To investigate the effect of bone marrow-derived monocytes transfected with RNA of CT-26 (a cell line of mouse colon carcinoma) on antitumor immunity.
METHODS: Mouse bone marrow-derived monocytes were incubated with mouse granulocyte macrophage colony stimulating factor (mGM-CSF) in vitro, and the purity of monocytes was detected by flow cytometry. Total RNA of CT-26 was obtained by TRIzol’s process, and monocytes were transfected by TransMessenger in vitro. The activity of cytotoxic T lymphocytes (CTL) in vivo was estimated by the modified lactate dehydrogenase (LDH) release assay. Changes of tumor size in mice and animal’s survival time were observed in different groups.
RESULTS: Monocytes from mouse bone marrow were successfully incubated, and the positive rate of CD11b was over 95%. Vaccination of the monocytes transfected with total RNA induced a high level of specific CTL activity in vivo, and made mice resistant to the subsequent challenge of parental tumor cells. In vivo effects induced by monocytes transfected with total RNA were stronger than those induced by monocytes pulsed with tumor cell lysates.
CONCLUSION: Antigen presenting cells transfected with total RNA of CT-26 can present endogenous tumor antigens, activate CTL, and effectively induce specific antitumor immunity.
- Citation: Chu XY, Chen LB, Zang J, Wang JH, Zhang Q, Geng HC. Effect of bone marrow-derived monocytes transfected with RNA of mouse colon carcinoma on specific antitumor immunity. World J Gastroenterol 2005; 11(5): 760-763
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/760.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.760
With the development of immunology, molecular biology, and genetic engineering technology, cancer immunotherapy has become the fourth mode following surgery, chemical therapy and radiotherapy. One of the hot spots in the field of tumor immunotherapy research is to develop a simple and effective specific tumor immunity[1]. Monocytes are important antigen presenting cells (APCs) in the body with the function of antigen processing and presenting. Monocytes activated by the antigen, mitogens and cytokines can transform into activated macrophages, and make the body produce a specific ability against tumors through activating T cells[2-5]. In this study, we observed the effects of bone marrow-derived monocytes transfected with total RNA of mice CT-26 on specific antitumor immunity in vivo.
Balb/c male mice, 6-8 wk old, were bought from Shanghai B and K Experimental Animal Co, Ltd. Mice colon carcinoma cell strain CT-26 was passaged routinely in our laboratory.
Reagents and suppliers were RPMI1640 (Gibco Company), fetal calf serum (Sijiqing Company of Hangzhou), IL-2 and mGM-CSF (Sigma Company), TransMessenger transfected reagent(Qiagen Company), CD11b-FITC fluorescent antibody (Pharmingen Company), LDH test reagent (Nanjing Jiancheng Reagent Company).
Mice femurs were taken in aseptic conditions and marrow cells were washed out, erythrocytes were dissolved with Tris-NH4Cl and washed twice with PBS. Then RPMI1640 culture medium with 10% fetal calf serum was added and its density was regulated to 1×106/mL, mGM-CSF 10 ng/mL was added and incubated in a cultural vial. On every fourth day the culture medium and floating cells were discarded, fresh medium and 10 ng/mL mGM-CSF were added. On the ninth day of culture in vitro, the supernate and floating cells were removed, the density was regulated and then the culture was added to a 6-well plate on the 6×106 /well, replenished with 2 mL fresh culture medium and 10 ng/mL mGM-CSF for each well and incubated overnight. The next step was to slightly blow its surface, and to discard all the culture medium and floating cells. Monocytes attached to the wall were harvested.
Monocytes harvested were added to 100 μL anti-CD11b antibody (its density 5 μg/mL) and incubated in darkness at 4 °C for 45 min. Monocytes were irrigated twice with PBS, then analyzed by a flow cytometer (FACS caliber, B.D Company).
After incubated for 24 h, total RNA of CT-26 cells was obtained by Trizol process when cells became confluent at 80%.The A260 and A280 values of total RNA were measured by spectrophotometer, and formaldehyde denatural electrophoresis was used to test its purity and integrity.
CT-26 cells were collected in vitro through passage culture for 24 h and washed twice with PBS, the density of which was regulated to 1×107/mL. The cells were frozen and thawed 4 times, centrifuged at 12000 g in low temperature for 30 min and prepared for use after the supernatant was removed and filtered.
Monocytes were transfected with total RNA in vitro according to the protocol of TransMessenger. The whole culture medium and floating cells in the 6-well plates were discarded. The monocytes attached to the wall were left and washed twice with PBS, 4 μL enhancer R was instilled to EPP tube with 94 μL buffer EC-R and then added to 2 μL RNA (RNA density 1 ng/μL), turned over and mixed evenly for 10 s. After keeping at room temperature for 5 min, the culture was centrifuged for 5 s, and then the supernate was removed and 8 μL TransMessenger reagent was added and again turned over and mixed evenly for 10 s. The culture was kept at room temperature for 10 min, then added to RPMI 1640 culture liquid without any calf serum and antibiotics. It was then vibrated twice and the tumor RNA-TransMessenger compounds were quickly instilled into the spare monocytes and the culture plate was lightly shaken. It was incubated at 37 °C in 50 mL/L CO2 for 3 h and then the supernate was removed, RPMI 1640 culture liquid was added and incubated for 24 h in an incubator. The next step was to add 10 mL EDTA and to assimilate the culture at room temperature for 5 min, and then monocytes were collected, and washed twice with PBS. Normal saline was added to regulate the monocyte density to 5×107/mL and immediately re-transfused to mice by injection into the abdominal cavity at 100 μL/mouse.
Monocytes were obtained by incubation as above, 20 μL tumor cell lysate was added and incubated at 37 °C in an incubator containing 50 mL/L CO2 for 24 h. After assimilated with EDTA, the monocytes were collected and washed twice with PBS. Normal saline was added to regulate the monocyte density to 5×107/mL, and then re-transfused to mice immediately by injection into the abdominal cavity at 100 μL/mouse.
The mice were randomly divided into 5 groups with 8 mice in each group. Abdominal cavity injection was performed at the dosage of 5×106/mouse. Group 1: monocytes transfected with tumor RNA by TransMessenger in vitro; group 2: monocytes plused with tumor RNA directly; group 3: monocytes plused with tumor lysates; group 4: monocytes; group 5: PBS. Subjects were inoculated with CT-26 cells at the dosage of 2×105 /mouse under the costal regions subcutaneously after 10 d of re-transfusion, and then the growth of tumor and the animal survival time were observed.
Cytotoxic activity was tested by LDH release assay[6]. Eight days after retransfusion of monocytes, 3 mice from each group were randomly selected and their spleens were taken to prepare cell suspension. Then 2×107 spleen cells were taken and added to 2×106 CT-26 cells that underwent inactivation for 40 min with mitomycin, 10 mL RPMI 1640, 10% fetal calf serum and IL-II 50 U/mL, and then incubated for 5 d at 37 °C 50 mL/L CO2. We collected live cells as the effector cells, mixed them with CT-26 target cells according to the effect/target ratios 100:1, 50:1 and 25:1, put in a 96 well-round bottom culture plate with its total volume reached to 200 μL, and incubated for 4 h. After incubation, 0.9% NaCl was added to stop effector/target reaction, and then centrifuged at 1500 r/min for 5 min and 100 μL supernate was taken to test LDH release rate. The killing activity of CTL cells against tumor cells was calculated with the following formula.
Killing activity (%)=(Sample release group A value--natural release group A value)/(The greatest release group A value--natural release group A value) ×100%
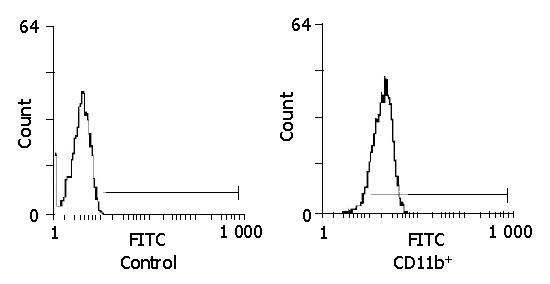
A lot of proliferated cell colonies attached to the wall could be obtained after mGM-CSF 10 ng/mL was added to mice bone marrow cells in vitro and incubated for 3-5 d. There were dead T and, B cells, erythrocytes and granulocytes in the supernate, which was removed when the liquid was changed. After culturing for 9 d, an abundance of proliferated cells was scattered in the supernate. These cells were removed and put into a new culture plate. After culturing overnight, granulocytes and dendritic cells were suspended in supernate with only monocytes attached to the wall. Only monocytes remained after the supernate was removed (Figure 1). Plenty of moncytes (over 108 cells) in the form of typical irregular stretching adherent cells were collected after culturing for 9 d in mouse bone marrow. These cells were sensitive to assimilation of EDTA and pancreatin, the specific mark of moncytes tested by flow cytometry was CD11b, and the positive rate of CD11b in moncytes was over 95% (Figure 2), which indicated that the purity of cultivated moncytes was quite high.
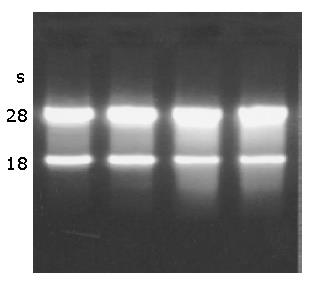
A260/280 of total RNA of CT-26 cells tested by spectrophotometer was 1.83, indicating that the purity of RNA was high. The results of formaldehyde denaturalization electrophoresis showed that the total RNA was free from DNA pollution. There were two bright bands, 28S and 18S, the proportion was 2:1 with no degradation band, indicating that the RNA had sound integrity and less degradation (Figure 3).
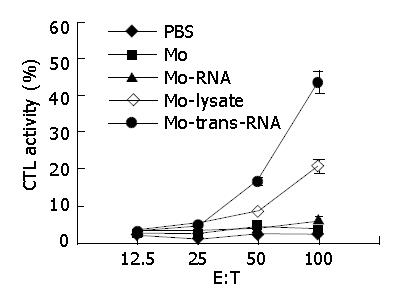
As shown in Figure 4, after monocytes transfected with total RNA of CT-26 cells in vitro by TransMessenger were re-transfused into the mice, the mice could be induced to generate high levels of activity of specific CTL. Monocytes impacted with CT-26 cell lysates in vitro could induce a moderate CTL activity in vivo. These results showed that monocytes transfected with tumor RNA in vitro by TransMessenger had a higher ability to activate CD8+CTL than those pulsed with tumor cell lysates in vitro.
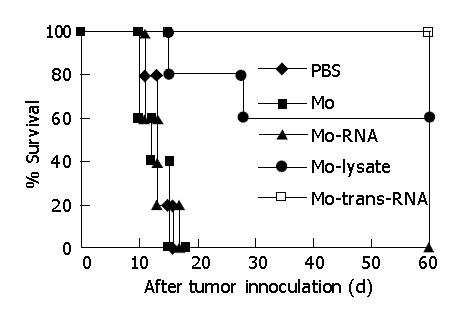
As shown in Figure 5, monocytes transfected with total RNA of CT-26 cells by TransMessenger could generate a stronger protecting immunity against attack by parent CT-26 tumor cells lately vaccinated. All mice could live longer and produce a completely protective effect because their immunologic function was raised in vivo. Retransfusion of monocytes pulsed with tumor cell lysates also generated a protective effect, but it was lower than that of the monocytes transfected with tumor total RNA, as only 60% of mice could survive for a long time.
T lymphocytes receive a specific antigen signal through TCR on its surface. That is, T lymphocytes receive a specific antigen signal through recognizing the compounds of MHC-I and MHC-II molecules on the surface of antigen presenting cells and tumor antigen peptide, which activate T lymphocytes with a specific subsidiary signal of antigen presenting cells stimulating molecules (B7, ICAM-1 and CD40) to bring the killing effect on tumor cells into play[7-9]. One of the main reasons for immune evasion in tumor cells is the lower ability of antigen presenting cells to present tumor antigen to T cells, which enable the body to generate effective antitumor immune reaction.
At present, tumor antigens include tumor cell lysates, withered corpuscles, irradiated and inactivated tumor vaccine, tumor antigen polypeptide, RNA coded tumor antigens and anti-specific tumor vaccines[10,11]. After these impacted antigen presenting cells in vitro are re-transfused to the subject mice, a strong antitumor immunological function can be produced[12-15], which demonstrates the clinical values for antitumor treatment. Monocytes transfected in vitro with tumor RNA that is taken as a kind of antigens could get endogenous expression antigens, and present MHC-I molecules to monocyte’s surface, which could activate CD8+ T cells, and when they are re-transfused to animals, the activity of lymphocytes on killing tumor in the body could be enhanced. As the professional antigen presenting cells, monocytes/macrophages play an important role in anti-tumor immune reaction[16]. But at present, it is a very complicated process to separate a large number of monocytes from peripheral blood and it is hard to meet the needs of clinical application. We took small amounts of bone marrow cells out of body and added cytokines for culturing and proliferating, and finally we obtained a large number of monocytes, which could be retransfused to the body to protect against tumor in vivo.
This study shows that when monocytes transfected with CT-26 cell total RNA by TransMessenger in vitro are re-transfused, they could induce a strong specific antitumor immune protection in vivo, demonstrating that tumor RNA extracted by TRIzol possesses good antigenic traits and pulsed monocytes in vitro could stimulate the body to produce antitumor ability markedly. Compared with other types of tumor antigens, the advantage of tumor RNA extracted by TRIzol is that it is easily prepared and could be used for every patient whose tumor cells could be obtained through operation or puncture. The extracted tumor RNA covers all the tumor antigens. TRIzol could also extract the RNA of specific tumor antigen effectively. Using the method of transfected medium, tumor RNA can be expressed endogenously as tumor antigens in antigen presenting cells, which could effectively activate the antitumor effect of T cells in the body.
In brief, monocytes transfected with tumor RNA in vitro by TransMessenger can be used as a new type of tumor vaccine, and they can induce a specific antitumor immunological function in vivo.
Edited by Wang XL Proofread by Chen WW
| 1. | Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 973] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 2. | Bonnotte B, Larmonier N, Favre N, Fromentin A, Moutet M, Martin M, Gurbuxani S, Solary E, Chauffert B, Martin F. Identification of tumor-infiltrating macrophages as the killers of tumor cells after immunization in a rat model system. J Immunol. 2001;167:5077-5083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Toujas L, Delcros JG, Diez E, Gervois N, Semana G, Corradin G, Jotereau F. Human monocyte-derived macrophages and dendritic cells are comparably effective in vitro in presenting HLA class I-restricted exogenous peptides. Immunology. 1997;91:635-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Yoshida R, Yoneda Y, Kuriyama M, Kubota T. IFN-gamma- and cell-to-cell contact-dependent cytotoxicity of allograft-induced macrophages against syngeneic tumor cells and cell lines: an application of allografting to cancer treatment. J Immunol. 1999;163:148-154. [PubMed] |
| 5. | Reiter I, Krammer B, Schwamberger G. Cutting edge: differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J Immunol. 1999;163:1730-1732. [PubMed] |
| 6. | Chen BY, Ma JY, Huang QT, You LF. Simplely natural killing experiment DA kind of modified LDH release assay. Shanghai Mianyixue Zazhi. 1989;9:218-219. |
| 7. | Zhan Y, Corbett AJ, Brady JL, Sutherland RM, Lew AM. CD4 help-independent induction of cytotoxic CD8 cells to allogeneic P815 tumor cells is absolutely dependent on costimulation. J Immunol. 2000;165:3612-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Tamura H, Dong H, Zhu G, Sica GL, Flies DB, Tamada K, Chen L. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Bai XF, Bender J, Liu J, Zhang H, Wang Y, Li O, Du P, Zheng P, Liu Y. Local costimulation reinvigorates tumor-specific cytolytic T lymphocytes for experimental therapy in mice with large tumor burdens. J Immunol. 2001;167:3936-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Koido S, Kashiwaba M, Chen D, Gendler S, Kufe D, Gong J. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. J Immunol. 2000;165:5713-5719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Gatza E, Okada CY. Tumor cell lysate-pulsed dendritic cells are more effective than TCR Id protein vaccines for active immunotherapy of T cell lymphoma. J Immunol. 2002;169:5227-5235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Henry F, Boisteau O, Bretaudeau L, Lieubeau B, Meflah K, Grégoire M. Antigen-presenting cells that phagocytose apoptotic tumor-derived cells are potent tumor vaccines. Cancer Res. 1999;59:3329-3332. [PubMed] |
| 13. | Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci USA. 2000;97:2715-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Ota S, Ono T, Morita A, Uenaka A, Harada M, Nakayama E. Cellular processing of a multibranched lysine core with tumor antigen peptides and presentation of peptide epitopes recognized by cytotoxic T lymphocytes on antigen-presenting cells. Cancer Res. 2002;62:1471-1476. [PubMed] |
| 15. | Strome SE, Voss S, Wilcox R, Wakefield TL, Tamada K, Flies D, Chapoval A, Lu J, Kasperbauer JL, Padley D. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 2002;62:1884-1889. [PubMed] |
| 16. | Mortarini R, Anichini A, Di Nicola M, Siena S, Bregni M, Belli F, Molla A, Gianni AM, Parmiani G. Autologous dendritic cells derived from CD34+ progenitors and from monocytes are not functionally equivalent antigen-presenting cells in the induction of melan-A/Mart-1(27-35)-specific CTLs from peripheral blood lymphocytes of melanoma patients with low frequency of CTL precursors. Cancer Res. 1997;57:5534-5541. [PubMed] |