Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.717
Revised: February 24, 2004
Accepted: March 18, 2004
Published online: February 7, 2005
AIM: To validate a radioactivity assay, the TCA-RA method, for the measurement of C-1027 in serum and to evaluate its application in determination of pharmacokinetics of C-1027 in mice.
METHODS: 125I-C-1027 was prepared by the Iodogen method and separated by HPLC. The radioactivity assay was established and used to determine 125I-C-1027 in mice at doses of 10, 50 and 100 μg/kg after precipitation with 20% trichloroacetic acid (TCA-RA method). Several pharmacokinetic parameters were determined after intravenous injection of 125I-C-1027 to mice.
RESULTS: After intravenous injection of 125I-C-1027 to mice, at doses of 10, 50 and 100 μg/kg; the apparent distribution volumes (Vd) were 0.26, 0.31 and 0.33 L/kg; the biological half-lives (T1/2) were 3.10, 3.40 and 3.90 h; the areas under curve (AUC) were 18.41, 103.69 and 202.74 ng/h/mL; the elimination rate constants (K) were 1.04, 1.26 and 0.58/h; and the total body clearance (Cl) were 0.54, 0.48 and 0.49 L/kg/h, respectively.
CONCLUSION: TCA-RA is a sensitive, reliable and suitable method for the determination of 125I-C-1027 in mouse serum.
- Citation: Liu YP, Li QS, Huang YR, Zhou MJ, Liu CX. Pharmacokinetics of C-1027 in mice as determined by TCA-RA method. World J Gastroenterol 2005; 11(5): 717-720
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/717.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.717
Lidamycin (C-1027), produced by Streptomyces globisporus in soil, consists of a non-covalently bound apoprotein, and a labile chromophore that is responsible for most of the biological activities[1-7]. The structure of C-1027 has been studied by several methods[8-10]. C-1027 shows a remarkable inhibition on the growth of human liver cancer, colon cancer and epithelial tumor cells[11-18], and exhibits highly potent cytotoxicity to cultured cancer cells and marked DNA cleaving ability[19-27]. The protein moiety of C-1027 has a single polypeptide chain cross-linked by two disulfide bonds with a molecular weight of 10 500 Da[28,29]. The protein protects the stability of the chromophore. Like other enediyne agents, antibiotic C-1027 is believed to exert its biological activity through the induction of cellular DNA and RNA damage[30-35]. The pre-clinical studies on the pharmacodynamics, pharmacokinetics and toxicology have demonstrated that C-1027 appears to be a very promising anticancer candidate, and has been used in clinical trial in China. The aim of this study was to validate a radioactivity assay after precipitation with 20% trichloroacetic acid (the TCA-RA method) for the measurement of serum C-1027 and to evaluate its application in determination of pharmacokinetics of C-1027 in mice.
C-1027 (Lot: 20020525, purity 95.0%) was produced by the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical University (Beijing, China). 125I-C-1027, which was radio-iodinated by the Iodogen method[36], had a specific radioactivity of 7.45 mCi (275.65 MBq)/mg. The radiochemical purity was more than 95%. Iodogen was from Academy of Military Medical Sciences (Beijing, China). Trichloroacetic acid (TCA, analytical grade) was provided by the Chemical Company (Beijing, China), and 0.9% sodium chloride was purchased from Dazhong Pharmaceutical Company (Tianjin, China). Distilled water, prepared from demineralized water, was used throughout the study. Gamma counter (FJ630G/12 model) was produced by Beijing Nuclear Company (Beijing, China). The chromatographic system (LC-6A, Shimadzu, Japan) consisted of a pump (LC-6AT), temperature box and variable wavelength UV detector (Spectra 100, Shimadzu, Japan). Sephadex G-50 column (300 mm×7.8 mm I.D) was purchased from Pharmacia Company (USA).
Kunming mice, male and female, with body weights from 17 to 24 g, were purchased from the Center of Experimental Animals of Tianjin Institute of Pharmaceutical Research (Certificate No: 20020804, Tianjin, China).
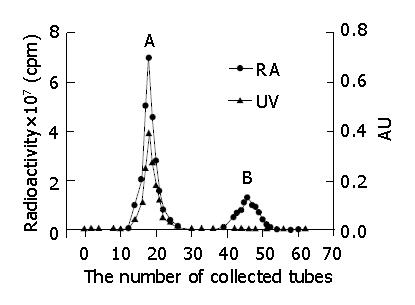
Iodogen (100 μg) in 100 μL of chloroform was placed in a sample tube, and evaporated to dryness with nitrogen gas. C-1027 (50 μg), and 50 μL of Na125I (74 MBq) were pipetted, mixed, and allowed to react at 15 °C for 30 min[36]. The mixture was chromatographed on Sephadex G-50 column. The mobile phase consisted of 0.05 mol/L sodium dodecyl sulfate phosphate buffer solution (pH 7.0) at a flow rate of 0.8 mL/min. The eluted fractions, detected by gamma counter as the same chromatographic behavior as standard C-1027 and Na125I, were components of 125I-C-1027 and Na125I, respectively (Figure 1). The fraction, collected from 10 min to 12.5 min, was a single radioactive peak of 125I-C-1027, and was concentrated to a specific activity and applied for pharmacokinetic study.
The radiochemical purity of 125I-C-1027 was calculated from the ratio of radioactivity of 125I-C-1027 to the collected total radioactivity. The biological activity of 125I-C-1027 was assayed in mice as previously described[37], and the biological activity was compared with C-1027. Only the 125I-C-1027, whose biological activity remained unchanged, was used to study the pharmacokinetics of 125I-C-1027.
Stock solution (10.0 μg/mL) of the 125I-C-1027 (275.65 KBq/mL) was prepared in water, and stored at -20 °C. The stock solution was prepared into the serial concentrations of standard solution of 0.5, 1.0, 2.0, 5.0, 10.0, 20.0, 50.0 and 100.0 ng/mL in 0.9% sodium chloride solution. The standard solution was used to prepare standard curves. The serial concentrations of calibration curves were prepared with mouse blank serum instead of 0.9% sodium chloride solution as mentioned above. Quality control (QC) samples were prepared into low, middle and high concentrations (0.5, 5.0 and 50 ng/mL) in mouse serum.
To 100 μL of mouse serum samples, 100 μL of 20% TCA was added. The mixture was vortexed for 2 min, and the supernatant was removed. Then, radioactivity of the precipitate was determined by the gamma counter.
Specificity of the assay was demonstrated by comparison between the radioactivity of the mouse serum spiked with 125I-C-1027 and the mouse blank serum.
QC samples of low, middle and high concentration levels (0.5, 5.0, and 50 ng/mL) were prepared for the determination of the precision and accuracy of intra- and inter-day. Precision, which was evaluated by one-way analysis (ANOVA), was defined as the relative standard deviation (RSD). Accuracy was defined as the relative errors (RE) between the measured and the nominal value on each of the three concentration levels.
Bench top stability was experimented at room temperature over 12 h. The QC samples in 6 replicates were analyzed at room temperature on the same day. Three freeze-thaw cycles were done on the QC samples.
Each sampling time was randomly distributed in 6 mice. Blood samples (0.4 mL) were collected at 0 min (pre-dose) and 2, 5, 15, 30 min and 1, 2, 4, 6, 8, 12, and 24 h after intravenous administration of 125I-C-1027 at doses of 10, 50, and 100 μg/kg. Serum samples were obtained by centrifuging at 2000 g for 10 min, and stored at -20 °C until analysis.
The concentration-time data were computed using a 3p97 Pharmacokinetic Calculation Program developed by the Mathematic Pharmacological Committee, Chinese Pharmacological Society (Beijing, China). The following pharmacokinetic parameters were calculated: biological half-life (T1/2), area under concentration-time curve (AUC), apparent distribution volume (Vd), the total body clearance (Cl), elimination rate constant (K), and other parameters.
The standard and calibration curve equations of 125I-C-1027 showed that the concentrations and their own radioactivity had a good linear correlation. The typical curve equations and correlation coefficients were as follows: y = 49.5 + 1235.9x (n = 8, r = 0.9994) for the standard solution of 125I-C-1027 at the concentration from 0.5 to 100.0 ng/mL, and y = 127.6 + 969.5x (n = 7, r = 0.9999) for serum samples from 0.5 to 100.0 ng/mL.
Precision and accuracy of the assay were evaluated by analyzing QC samples (0.5, 5.0 and 50.0 ng/mL) in 6 replicates on 3 different days. The relative standard deviation (RSD) was less than 5.0% for intra-day assay and less than 10.1% for inter-day assay at 0.5-100.0 ng/mL. The accuracy was between 96.0% and 99.0% (Table 1). The limit of quantitation was the lowest concentration on the calibration curve if the following conditions were met. (1) There was no interference present in blanks at the retention time of the analyte, or the determination response was at least 10 times greater than any interference in blank sample at the retention time; (2) Analyte peak should be identifiable, discrete and reproducible with a precision of less or equal to 15% and accuracy within ±15%. The limit of quantitation of 125I-C-1027 for the TCA-RA method was 0.5 ng/mL.
| Added (ng/mL) | Within-day | Between-day | ||||
| Found | RSD (%) | Accuracy (%) | Found | RSD (%) | Accuracy (%) | |
| 1 | 0.98±0.05 | 5 | 98 | 0.96±0.04 | 4.6 | 96 |
| 2.5 | 2.47±0.05 | 1.9 | 98.6 | 2.45±0.25 | 10.1 | 98.2 |
| 20 | 9.81±0.14 | 0.7 | 99 | 19.65±1.00 | 5.1 | 98.4 |
The bench top stability of 125I-C-1027 in mouse serum was determined over 12 h at room temperature. The results showed that 125I-C-1027 was stable at the concentrations (0.5, 5.0 and 50.0 ng/mL) at room temperature (more than 92.0%, 91.0% and 90.0%, respectively). Similarly when 125I-C-1027 underwent 3 freeze-thaw cycles, the percentage differences were 8.9%, 7.3% and 8.2% at 3 different concentrations, respectively.
The comparison of blank serum samples and serum samples spiked with 125I-C-1027 showed no endogenous interference with the measurement of 125I-C-1027.
The validation of the TCA-RA method satisfied the requirements for bioanalysis[38-42].
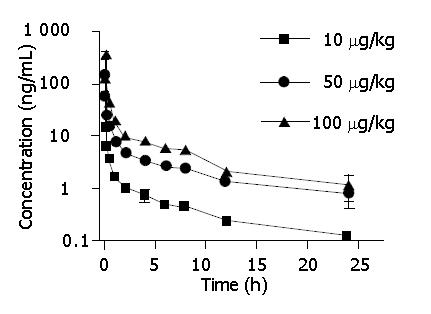
After intravenous injection of 10, 50 and 100 μg/kg 125I-C-1027 to mice, the serum concentrations of 125I-C-1027 were determined by the TCA-RA method. Figure 2 shows the serum concentration-time curve of 125I-C-1027 after intravenous administration (n = 6). The results of this experiment showed that the pharmacokinetic parameters: T1/2, Cl, Vd and K did not exhibit statistically significant differences (P>0.05) among the three doses, and the AUC values depended on the administration dose (Table 2).
| Parameters | Unit | Doses (mg/kg) | P | ||
| 10 | 50 | 100 | |||
| A | ng/mL | 34.62 | 142.98 | 277.02 | |
| a | /h | 9.61 | 9.64 | 4.92 | |
| B | ng/mL | 3.31 | 18.1 | 26.02 | |
| b | /h | 0.22 | 0.2 | 0.18 | |
| V(d) | L/kg | 0.26 | 0.31 | 0.33 | >0.05 |
| T1/2a | h | 0.07 | 0.07 | 0.14 | |
| T1/2b | h | 3.1 | 3.4 | 3.9 | >0.05 |
| K21 | /h | 1.04 | 1.26 | 0.58 | |
| K10 | /h | 2.06 | 1.55 | 1.49 | >0.05 |
| K12 | /h | 6.73 | 7.03 | 3.02 | |
| AUC | ng.h/mL | 18.41 | 103.69 | 202.74 | |
| CL(S) | L/(kg.h) | 0.54 | 0.48 | 0.49 | >0.05 |
Biotechnological pharmaceuticals can be analyzed by many methods, such as bioassays, immunoassays, enzyme-linked immunosorbent assay and solid-phase radioimmunoassay. However, these methods are limited due to the interference of endogenous substances. On the other hand, isotope labeling methods used to analyze pharmacokinetic properties of biotechnological products, can eliminate the interference of endogenous substances, and improve the specificity, accuracy, limit of quantitation, and the speed of analysis.
The method of 125I labeling C-1027 is simple, quick and acceptable. The highly purified 125I-C-1027 (with purity of more than 95.0%) is obtained by the Sephadex G-50 gel filtration, indicating that Sephadex G-50 gel filtration is an effective procedure to yield the high quality 125I-labeled C-1027. Only the 125I-C-1027 with purity and biological activity in accord ance with the regulation can be used for the pharmacokinetic experiments in mice.
The TCA-RA method has been considered to be an accepted method for assay of the precipitated 125I-C-1027[43]. In this study, the mouse serum determination and pharmacokinetic profiles of 125I-C-1027 were measured by the TCA-RA method after intravenous injection of 10, 50, and 100 µg/kg 125I-C-1027 to mice. The pharmacokinetic results show that the areas under curves of three doses (10, 50, and 100 µg/kg) depend on the doses. The biological half-lives (T1/2) do not change with the doses. The limit of quantitation indicates that this method is a sensitive method for analysis of C-1027.
The authors are indebted to the Institute of Medicinal Biotechnology (Beijing, China) for their financial support during the course of the study.
Edited by Xia HHX and Wang XL Proofread by Chen WW
| 1. | Hu JL, Xue YC, Xie MY, Zhang R, Otani T, Minami Y, Yamada Y, Marunaka T. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J Antibiot (Tokyo). 1988;41:1575-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Otani T, Minami Y, Marunaka T, Zhang R, Xie MY. A new macromolecular antitumor antibiotic, C-1027. II. Isolation and physico-chemical properties. J Antibiot (Tokyo). 1988;41:1580-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Zhen YS, Ming XY, Yu B, Otani T, Saito H, Yamada Y. A new macromolecular antitumor antibiotic, C-1027. III. Antitumor activity. J Antibiot (Tokyo). 1989;42:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Otani T, Yasuhara T, Minami Y, Shimazu T, Zhang R, Xie MY. Purification and primary structure of C-1027-AG, a selective antagonist of antitumor antibiotic C-1027, from Streptomyces globisporus. Agric Biol Chem. 1991;55:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Liu W, Shen B. Genes for production of the enediyne antitumor antibiotic C-1027 in Streptomyces globisporus are clustered with the cagA gene that encodes the C-1027 apoprotein. Antimicrob Agents Chemother. 2000;44:382-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Matsumoto T, Okuno Y, Sugiura Y. Specific interaction between a novel enediyne chromophore and apoprotein in macromolecular antitumor antibiotic C-1027. Biochem Biophys Res Commun. 1993;195:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Otani T. Conformation studies on and assessment by spectral analysis of the protein-chromophore interaction of the macromolecular antitumor antibiotic C-1027. J Antibiot (Tokyo). 1993;46:791-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Chen Y, Shao R, Bartlam M, Li J, Jin L, Gao Y, Liu Y, Tang H, Zhen Y, Rao Z. Crystallization and preliminary X-ray crystallographic studies of a macromolecular antitumour antibiotic, C1027. Acta Crystallogr D Biol Crystallogr. 2002;58:173-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Tanaka T, Fukuda-Ishisaka S, Hirama M, Otani T. Solution structures of C-1027 apoprotein and its complex with the aromatized chromophore. J Mol Biol. 2001;309:267-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Semmelhack MF, Jiang Y, Ho D. Synthesis of the amino sugar from C-1027. Org Lett. 2001;3:2403-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Xu YJ, Li DD, Zhen YS. Mode of action of C-1027, a new macromolecular antitumor antibiotic with highly potent cytotoxicity, on human hepatoma BEL-7402 cells. Cancer Chemother Pharmacol. 1990;27:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | He QY, Liang YY, Wang DS, Li DD. Characterization of cell death induced by anticancer antibiotic lidamycin in human hepatoma BEL-7402 cells. Yaoxue Xuebao. 2001;36:174-178. |
| 13. | Cui DP, Wang Z, Li DD. Effect of lidamycin on the expression of genes involved in invasion regulation in HCT-8 human colon cancer cells. Yaoxue Xuebao. 2001;36:246-249. |
| 14. | He QY, Jiang B, Li DD, Zhen YS. Effects of lidamycin on apoptotic gene expressions and cytoskeleton in human hepatoma bel-7402 cells. AiZheng. 2002;21:351-355. [PubMed] |
| 15. | He QY, Jiang B, Li DD. Effects of lidamycin on genomic DNA in human hepatoma BEL-7402 cells. Acta Pharmacol Sin. 2002;23:253-256. [PubMed] |
| 16. | He QY, Liang YY, Wang DS, Li DD. Characteristics of mitotic cell death induced by enediyne antibiotic lidamycin in human epithelial tumor cells. Int J Oncol. 2002;20:261-266. [PubMed] |
| 17. | Zhen H, Xue Y, Zhen Y. Inhibition of angiogenesis by antitumor antibiotic C1027 and its effect on tumor metastasis. Zhonghua YiXue ZaZhi. 1997;77:657-660. [PubMed] |
| 18. | Li J, Zhen Y, Yang Z. Biodistribution of monoclonal antibody and Fab fragment and antitumor effect of their conjugates on hepatoma xenografts. Zhongguo YiXueKeXueYuan XueBao. 1994;16:328-333. [PubMed] |
| 19. | Sugiura Y. Molecular mechanisms of DNA recognition and function by bioactive compounds. Yakugaku Zasshi. 2000;120:1409-1418. [PubMed] |
| 20. | Hiraku Y, Oikawa S, Kawanishi S. Distamycin A, a minor groove binder, changes enediyne-induced DNA cleavage sites and enhances apoptosis. Nucleic Acids Res Suppl. 2002;95-96. [PubMed] |
| 21. | Wang Z, He Q, Liang Y, Wang D, Li YY, Li D. Non-caspase-mediated apoptosis contributes to the potent cytotoxicity of the enediyne antibiotic lidamycin toward human tumor cells. Biochem Pharmacol. 2003;65:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Dziegielewski J, Beerman TA. Cellular responses to the DNA strand-scission enediyne C-1027 can be independent of ATM, ATR, and DNA-PK kinases. J Biol Chem. 2002;277:20549-20554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | McHugh MM, Yin X, Kuo SR, Liu JS, Melendy T, Beerman TA. The cellular response to DNA damage induced by the enediynes C-1027 and neocarzinostatin includes hyperphosphorylation and increased nuclear retention of replication protein a (RPA) and trans inhibition of DNA replication. Biochemistry. 2001;40:4792-4799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | McHugh MM, Beerman TA. C-1027-induced alterations in Epstein-Barr viral DNA replication in latently infected cultured human Raji cells: relationship to DNA damage. Biochemistry. 1999;38:6962-6970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Kirk CA, Goodisman J, Beerman TA, Gawron LS, Dabrowiak JC. Kinetics of cleavage of intra- and extracellular simian virus 40 DNA with the enediyne anticancer drug C-1027. Biophys Chem. 1997;63:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Matsumoto T, Sugiura Y. Alterations of binding mode and cutting site by G--& gt; I replacement in preferred cleavage sequences 5'-AGG of chromoprotein C-1027. Biochem Biophys Res Commun. 1994;205:1533-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Xu YJ, Zhen YS, Goldberg IH. C1027 chromophore, a potent new enediyne antitumor antibiotic, induces sequence-specific double-strand DNA cleavage. Biochemistry. 1994;33:5947-5954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Zhou CS, Xu LN, Jiang M, Zhen YS. A monoclonal antibody directed against an enediyne antitumor antibiotic and its preliminary application. Yaoxue Xuebao. 1997;32:28-32. |
| 29. | Shao RG, Zhen YS. Relationship between the molecular composition of C1027, a new macromolecular antibiotic with enediyne chromophore, and its antitumor activity. Yaoxue Xuebao. 1995;30:336-342. |
| 30. | Liu JS, Kuo SR, Yin X, Beerman TA, Melendy T. DNA damage by the enediyne C-1027 results in the inhibition of DNA replication by loss of replication protein A function and activation of DNA-dependent protein kinase. Biochemistry. 2001;40:14661-14668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Xu YJ, Xi Z, Zhen YS, Goldberg IH. Mechanism of formation of novel covalent drug.DNA interstrand cross-links and monoadducts by enediyne antitumor antibiotics. Biochemistry. 1997;36:14975-14984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Sugiura Y, Totsuka R, Araki M, Okuno Y. Selective cleavages of tRNAPhe with secondary and tertiary structures by enediyne antitumor antibiotics. Bioorg Med Chem. 1997;5:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | McHugh MM, Beerman TA, Burhans WC. DNA-damaging enediyne C-1027 inhibits initiation of intracellular SV40 DNA replication in trans. Biochemistry. 1997;36:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Xu YJ, Xi Z, Zhen YS, Goldberg IH. A single binding mode of activated enediyne C1027 generates two types of double-strand DNA lesions: deuterium isotope-induced shuttling between adjacent nucleotide target sites. Biochemistry. 1995;34:12451-12460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Totsuka R, Aizawa Y, Uesugi M, Okuno Y, Matsumoto T, Sugiura Y. RNA cleavage by C-1027 chromophore, an enediyne antitumor antibiotic: high selectivity to an anticodon arm. Biochem Biophys Res Commun. 1995;208:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Tang ZM, Liu XW, Xu LP, Shan CW, Song QS. Pharmacokinetics and tissue distribution of human recombinant interleukin-2 in mice. Zhongguo Yao Li Xue Bao. 1994;15:51-56. [PubMed] |
| 37. | Sugimoto Y, Otani T, Oie S, Wierzba K, Yamada Y. Mechanism of action of a new macromolecular antitumor antibiotic, C-1027. J Antibiot (Tokyo). 1990;43:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Liu CX, Wei GL, Li QS. Methodology study of validation for bioanalysis in studies on pharmacokinetics and bioavailability. Asian J Drug Metab Pharmacokinet. 2001;1:279-286. |
| 39. | Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Cook CE, McDowall RD. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J Drug Metab Pharmacokinet. 1991;16:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 382] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 40. | Gao J. Bioanalytical method validation for studies on pharmacokinetics, bioavailability and bioequivalence: Highlights of the FDA’s Guidance. Asian J Drug Metab Pharmacokinet. 2004;4:5-13. |
| 41. | Xia JH. Validation of analytical methods for pharmacokinetics, boiavailability and bioequivalence studies. Asian J Drug Metab Pharmacokinet. 2001;1:95-100. |
| 42. | Liu YP, Zhou MJ. Effect of polyethylene glycol derivation on pharmacokinetic properties of biotechnical drugs. Asian J Drug Metab Pharmacokinet. 2002;2:127-131. |