Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.623
Revised: May 14, 2004
Accepted: June 17, 2004
Published online: February 7, 2005
AIM: To investigate the expression of mitogen-activated protein kinases (MAPKs) and its upstream protein kinase in human gastric cancer and to evaluate the relationship between protein levels and clinicopathological parameters.
METHODS: Western blot was used to measure the expression of extracellular signal-regulated kinase (ERK)-1, ERK-2, ERK-3, p38 and mitogen or ERK activated protein kinaseMEK-1 proteins in surgically resected gastric carcinoma, adjacent normal mucosa and metastatic lymph nodes from 42 patients. Immunohistochemistry was employed for their localization.
RESULTS: Compared with normal tissues, the protein levels of ERK-1 (integral optical density value 159526±65760 vs 122807±65515, P = 0.001), ERK-2 (168471±95051 vs 120469±72874, P<0.001), ERK-3 (118651±71513 vs 70934±68058, P<0.001), P38 (104776±51650 vs 82930±40392, P = 0.048) and MEK-1 (116486±45725 vs 101434±49387, P = 0.027) were increased in gastric cancer tissues. Overexpression of ERK-3 was correlated to TNM staging [average ratio of integral optic density (IOD)tumor: IODnormal in TNM I, II, III, IV tumors was 1.43±0.34, 5.08±3.74, 4.99±1.08, 1.44±1.02, n = 42, P = 0.023] and serosa invasion (4.31±4.34 vs 2.00±2.03, P = 0.037). In poorly differentiated cancers (n = 33), the protein levels of ERK-1 and ERK-2 in stage III and IV tumors were higher than those in stage I and II tumors (2.64±3.01 vs 1.01±0.33, P = 0.022; 2.05±1.54 vs 1.24±0.40, P = 0.030). Gastric cancer tissues with either lymph node involvement (2.49±2.91 vs 1.03±0.36, P = 0.023; 1.98±1.49 vs 1.24±0.44, P = 0.036) or serosa invasion (2.39±2.82 vs 1.01±0.35, P = 0.022; 1.95±1.44 vs 1.14±0.36, P = 0.015) expressed higher protein levels of ERK-1 and ERK-2. In Borrmann II tumors, expression of ERK-2 and ERK-3 was increased compared with Borrmann III tumors (2.57±1.86 vs 1.23±0.60, P = 0.022; 5.50±5.05 vs 1.83±1.21, P = 0.014). Borrmann IV tumors expressed higher p38 protein levels. No statistically significant difference in expression of MAPKs was found when stratified to tumor size or histological grade (P>0.05). Protein levels of ERK-2, ERK-3 and MEK-1 in metastatic lymph nodes were 2-7 folds higher than those in adjacent normal mucosa. The immunohistochemistry demonstrated that ERK-1, ERK-2, ERK-3, p38 and MEK-1 proteins were mainly localized in cytoplasm. The expression of MEK-1 in gastric cancer cells metastasized to lymph nodes was higher than that of the primary site.
CONCLUSION: MAPKs, particularly ERK subclass are overexpressed in the majority of gastric cancers. Overexpression of ERKs is correlated to TNM staging, serosa invasion, and lymph node involvement. The overexpression of p38 most likely plays a prominent role in certain morphological subtypes of gastric cancers. MEK-1 is also overexpressed in gastric cancer, particularly in metastatic lymph nodes. Upregulation of MAPK signal transduction pathways may play an important role in tumorigenesis and metastatic potential of gastric cancer.
- Citation: Liang B, Wang S, Zhu XG, Yu YX, Cui ZR, Yu YZ. Increased expression of mitogen-activated protein kinase and its upstream regulating signal in human gastric cancer. World J Gastroenterol 2005; 11(5): 623-628
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/623.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.623
The incidence and mortality of gastric cancer have decreased markedly since 1930s[1]. This decrease is mirrored worldwide, but it is comparatively higher in Japan, China, Chile, and Ireland. Gastric cancer still is the leading cause of cancer-related deaths in China[2].
Surgical removal with resection of adjacent lymph nodes offers the only chance for cure, which is less than 33% of patients with gastric cancer. The five-year survival rate is 30-40% with a poorer prognosis of advanced tumors[3-5]. Thus far, current treatments have largely been unsuccessful. Therefore it is critical to further elucidate the etiological factors and molecular mechanism for the pathogenesis of gastric cancer.
Mitogen-activated protein kinases (MAPKs) are serine/threonine kinases activated in response to a variety of external signals. Three major subclasses of MAPKs, namely, extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 have been identified[6,7]. Various receptor tyrosine kinases, cytokine receptors, G proteins and oncogene products can activate MAPKs through phosphorylation by mitogen activated protein kinase or ERK-activated protein kinase (MEK)[8-10]. Thus, MAPKs are proposed to be a critical integrator of various signaling transduction systems and involved in various cellular processes including cell proliferation, differentiation, apoptosis, and transformation[6,7]. Constitutive activation of these signaling cascades has been noted in the malignant transformation of various cell lines[11,12] and implicated in carcinogenesis and metastatic potential of human cancers[13-15]. The purpose of this study was to investigate the expression of MAPKs and its upstream regulating signals in human gastric cancer and to evaluate the relationship between protein levels and clinicopathological parameters.
Immobilon P PVDF membranes for Western blot were purchased from Millipore (Bedford, MA), and X-ray film was purchased from Eastman Kodak (Rochester, NY). Antibodies to ERK-l (sc-94), ERK-2 (sc-154), ERK-3 (sc-155), p38 (sc-535) and MEK-1 (sc-219) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The enhanced chemiluminescence (ECL) system for Western immunoblot analysis was purchased from Amersham (Arlington Heights, IL). The concentrated protein assay dye reagent was purchased from Bio-Rad Laboratories (Hercules, CA). All other reagents were of molecular biology grade and purchased from either Sigma or Amresco (Solon, OH).
Primary gastric adenocarcinomas, adjacent (5 to 10 cm from the primary site) normal mucosa and metastatic lymph nodes were obtained from 42 continuously enrolled patients undergoing elective radical gastrectomy at the Peking University People’s Hospital from July 1998 to January 2000. Resected tissues were immediately flash frozen with liquid nitrogen. Tumors were classified according to the histological subgroups recommended by the World Health Organization (WHO) and staged by the TNM system.
Tissues were homogenized on ice in RIPA lysis buffer (50 mmol/L NaCL, 50 mmol/L Tris, pH 7.4, 0.5% NP-40, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L NaF, 1 ug/mL aprotinin, 1 ug/mL leupeptin). Cell extracts were then clarified by centrifugation (for 30 min at 12000 g) and protein concentration was determined using the method of Bradford[16].
Western blotting was performed as described previously[17]. Briefly, whole cell lysates (50 ug) were denatured in SDS sample loading buffer (50 mmol/L Tris, pH 6.8, 100 mmol/L DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol) and fractionated on a 10% SDS-polyacrylamide gel. Proteins were electroblotted to Immobilon-P PVDF membranes. Filters were probed overnight at 4 °C in blocking solution (TBS containing 5% non-fat dried milk, 0.1% Tween 20) followed by an incubation for 3 h with the primary antibody. Filters were then washed in blocking solution and incubated with a horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G (IgG) as a secondary antibody for 1 h. After 4 final washes, the immune complexes were visualized using ECL detection. ERK-1, ERK-2, ERK-3, p38 and MEK-1 expression in tumor and paired normal mucosae were quantitated by densitometry to obtain an integral optic density (IOD) value.
In brief, sections (5 μm thick) placed on silane-coated slides (Muto Pure Chemicals, Sigma, Co. USA) were deparaffinized, rehydrated and then pretreated with 3% H2O2 in methanol for 20 min at 25 °C to quench the endogenous peroxidase activity. The sections were then placed in 10 mmol/L citrate buffer (pH 6.0) and heated in a microwave oven (400 W) for 10 min to facilitate antigen retrieval. Subsequently, the sections were incubated with 10% goat non-immune serum-3% BSA in PBS at room temperature for 60 min to block the nonspecific binding and then incubated with primary antibodies for 1 h. The sections were then incubated at room temperature with the secondary antibodies (biotinylated goat anti-rabbit IgG; Dako, Denmark) followed by incubation with avidin-biotin-peroxidase complex (Dako, Denmark), labeled with peroxidase and colored with diaminobenzidine substrate. Sections of gastric adenocarcinoma, confirmed to overexpress the protein of interest, were used as positive controls. No primary antibody was used as negative control.
Paired-samples t test was used to analyze the difference in MAPKs expression between cancer tissues and normals. Either independent-sample t test or one-way ANOVA was used to analyze the relationships between IODtumor/IODnormal ratios and clinicopathological characteristics. All analyses were performed with the SPSS statistical software (Ver. 9.0). P values less than 0.05 were considered statistically significant.
To determine whether ERK-1, ERK-2 or ERK-3 was overexpressed in gastric cancer, 42 gastric cancer tissues and adjacent normal mucosa were examined by Western blot. The protein levels of ERK-1, ERK-2 and ERK-3 were increased in gastric cancer tissues compared with adjacent normal mucosa (P<0.001, Table 1). ERK-1, ERK-2 and ERK-3 were overexpressed, as defined by an IODtumor/IODnormal ratio >1.3, in 22, 22 and 27 cases of gastric cancer, respectively (Figure 1). The average protein levels of ERK-1, ERK-2 and ERK-3 in gastric cancer were 2.10, 2.04 and 3.85 folds higher than those in adjacent normal mucosa, respectively. Because parts of our samples were used up in previous experiments, the expressions of p38 and MEK-1 were detected in 30 gastric cancers. The protein levels of p38 (P = 0.048) and MEK-1 (P = 0.027, Table 1) were increased in gastric cancer tissues compared with adjacent normal mucosa. MEK-1 and p38 were overexpressed in 14 and 12 cases of gastric cancer, respectively (Figure 1). The average protein levels of p38 and MEK-1 in gastric cancer were 1.71 and 1.31 folds higher than those in adjacent normal mucosa, respectively.
| Items | n | IOD value | t | P | |
| Gastric cancer | Normal mucosa | ||||
| ERK-1 | 42 | 159526±65760 | 122807±65515 | 3.658 | 0.001 |
| ERK-2 | 42 | 168471±95051 | 120469±72874 | 4.758 | <0.001 |
| ERK-3 | 42 | 118651±71513 | 70934±68058 | 5.065 | <0.001 |
| P38 | 30 | 104776±51650 | 82930±40392 | 2.064 | 0.048 |
| MEK-1 | 30 | 116486±45725 | 101434±49387 | 2.16 | 0.027 |
When stratified to clinicopathological parameters, expression of ERK-2 and ERK-3 was be increased in Borrmann II tumors in comparison to Borrmann III or Borrmann IV tumors (2.57±1.86 vs 1.23±0.60, P = 0.022; 5.50±5.05 vs 1.83±1.21, P = 0.014). The protein levels of ERK-3 were higher in stage II and stage III tumors than those in stage I and IV tumors (average ratio of IODtumor:IODnormals in TNM I, II, III, IV tumors was 1.43±0.34, 5.08±3.74, 4.99±1.08, 1.44±1.02, n = 42, P = 0.023). Stage III tumors trended to express higher levels of ERK-1 and ERK-2 proteins, but no statistically significant difference was found (P>0.05). Gastric cancer tissues with serosa invasion expressed higher protein levels of ERK-3 (4.31±4.34 vs 2.00±2.03, P = 0.037).
Interestingly, in 33 cases of poorly differentiated cancer, the protein levels of ERK-1 and ERK-2 in stage III and IV tumors were higher than those in stage I and II tumors (Table 2, P<0.05). Gastric cancer tissues with either lymph node metastasis or serosa invasion expressed higher protein levels of ERK-1 and ERK-2 (P<0.05). No statistically significant difference in ERK expression was found when stratified to tumor size or histological grade (P>0.05).
| Items | n | ERK1 | ERK2 | ||||
| T:N ratio1 | F (t) | P | T:N ratio1 | F (t) | P | ||
| TNM stage | |||||||
| I, II | 11 | 1.01±0.33 | 2.505 | 0.022 | 1.24±0.40 | 2.297 | 0.03 |
| III, IV | 22 | 2.64±3.01 | 2.05±1.54 | ||||
| Lymph node involvement | |||||||
| + | 24 | 2.49±2.91 | 2.421 | 0.023 | 1.98±1.49 | 2.191 | 0.036 |
| - | 9 | 1.03±0.36 | 1.24±0.44 | ||||
| Serosa invasion | |||||||
| + | 26 | 2.39±2.82 | 2.428 | 0.022 | 1.95±1.44 | 2.585 | 0.015 |
| - | 7 | 1.01±0.35 | 1.14±0.36 | ||||
| Tumor size | |||||||
| <5 cm | 19 | 1.97±2.30 | 0.323 | 0.749 | 1.72±1.31 | 0.309 | 0.76 |
| >5 cm | 14 | 2.27±2.96 | 1.86±1.39 | ||||
| Borrmann classification | |||||||
| I | 5 | 1.27±0.53 | 1.42 | 0.257 | 0.96±0.22 | 1.169 | 0.338 |
| II | 22 | 2.17±1.64 | 2.96±3.27 | ||||
| III | 12 | 1.30±0.68 | 1.02±0.37 | ||||
| IV | 3 | 1.38±0.19 | 1.59±0.20 | ||||
No statistically significant association was found between the IODtumor/IODnormal ratio of p38 or MEK-1 proteins when stratified to age, sex, histological classification, or TNM staging of patients. However in Borrmann IV cancers, there was an increase in p38 expression compared with Borrmann II (3.83±4.43 vs 1.64±1.21, P = 0.039) and Borrmann III tumors (3.83±4.43 vs 1.19±0.89, P = 0.019), as defined by the average ratio of IODtumor: IODnormal.
Although the cellular content of metastatic lymph nodes was heterogenous, we found that the overall expression of ERK-2, and ERK-3 was still increased (Figure 1 and Table 3). The average protein levels of ERK-2 and ERK-3 in metastatic lymph nodes were 1.68 and 6.89 folds higher than those in adjacent normal mucosa in 14 paired samples, respectively.
| Items | n | IOD value | t | P |
| ERK-1 | ||||
| Metastatic lymph nodes | 14 | 195734.7±70508.8 | ||
| Primary site | 210423.7±74207.3 | 0.608 | 0.554b | |
| Normal mucosa | 155862.3±79052.8 | 1.453 | 0.170a | |
| ERK-2 | ||||
| Metastatic lymph nodes | 14 | 186959.5±80801.8 | ||
| Primary site | 211155.1±64116.8 | 0.893 | 0.388b | |
| Normal mucosa | 127092.4±56585.6 | 2.579 | 0.023a | |
| ERK-3 | ||||
| Metastatic lymph nodes | 14 | 140323.2±120532.0 | ||
| Primary site | 98732.1±54021.3 | 1.261 | 0.230b | |
| Normal mucosa | 50032.9±58937.8 | 3.116 | <0.001a | |
| P38 | ||||
| Metastatic lymph nodes | 12 | 96866.8±76857.3 | ||
| Primary site | 99740.6±38279.6 | 0.202 | 0.844b | |
| Normal mucosa | 77784.4±30367.0 | 0.797 | 0.442a | |
| MEK-1 | ||||
| Metastatic lymph nodes | 12 | 133353.9±42374.3 | ||
| Primary site | 101112.5±39124.0 | 2.203 | 0.05b | |
| Normal mucosa | 77431.9±31282.1 | 4.235 | <0.001a |
Metastatic lymph nodes expressed higher levels of MEK-1 protein when compared with normal mucosa and primary sites (P<0.05, Figure 1 and Table 3). The average protein level of MEK-1 in metastatic lymph nodes was 2.09 folds higher than that in adjacent normal mucosa in 12 paired samples. The protein level of p38 was not increased in metastatic lymph nodes in comparison to normal mucosa or primary sites (P>0.05).
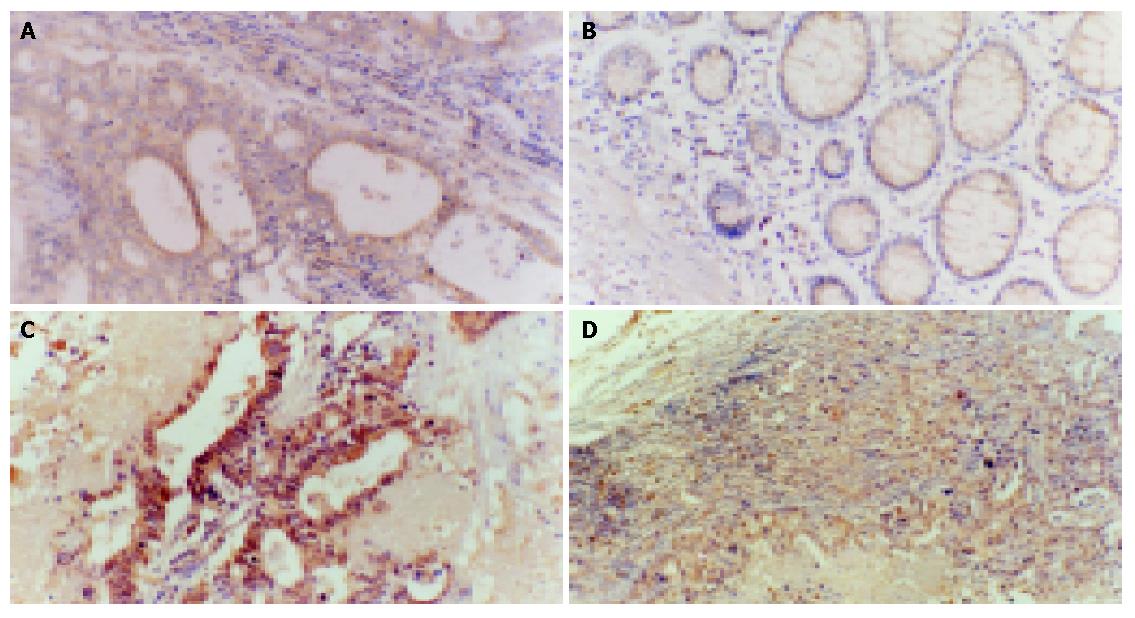
Immunohistochemistry of gastric carcinomas and normal mucosa demonstrated that ERK-1, ERK-2, ERK-3, p38 and MEK1 proteins were localized in the cytoplasm. Gastric cancer cells expressed higher levels of these proteins compared with adjacent normal mucosa. In particular, the expression of MEK-1 in metastatic gastric cancer cells localized in lymph nodes was higher than that of the primary sites (Figures 2A-D).
Mitogen-activated protein kinase is a key molecule in intracellular signal transducing pathways that transport extracellular stimuli from cell surface to nuclei. MAPK pathway has been revealed to be involved in the physiological proliferation of mammalian cells and also to potentiate them to transform[6,7]. With regard to human gastric carcinoma, investigations of alterations in expression and activity of components of the MAPK cascade will help to understand the mechanisms of malignant behaviors of gastric cancer cells.
In the present report, the expression of MAPKs was examined by Western blot in 42 gastric cancer tissues and adjacent normal mucosa. Our results demonstrate that the protein levels of ERK-1, ERK-2 and ERK-3 are increased in gastric cancer tissues compared with adjacent normal mucosa. The average protein levels of ERK-1, ERK-2 and ERK-3 in gastric cances are 2.10, 2.04 and 3.85 folds higher than those in adjacent normal mucosa, respectively. In a previous report[18], we showed an increase of phosphorylated ERK1/2 in gastric adenocarcinoma compared to normals and the consistency of ERK1/2 activity and expression. Therefore, our results suggest that ERK hyperexpression and increased activation may contribute to the determination of the constitutive activation of MAPK/ERK pathways in gastric carcinoma. Iwase et al[19] found that upregulation of ERK pathway could contribute to proliferation and transformation of gastric mucosa cells. Thus constitutive activation of MAPK/ERK pathways may play an important role in tumorigenesis of gastric cancer.
Cancer development is thought to be regulated by the integration of distinct signals. It has been reported that ERK-3 is structurally related to the better characterized ERK-1 and ERK-2 with 43% overall homology[20], however little is known about its cellular function. Recent studies have shown that activation of ERK-3 may participate in recovery of malignant behaviors of colon HD3 cell line, including tumorigenesis in nude mice[21]. Wang et al[22] found that the activation of ERK-3 was increased in colon cancer, but the relationship between ERK-3 activation and clinicopathological characteristics remains to be identified. Our results demonstrate that overexpression of ERK-3 is noted in 64.3% samples, and increased protein levels of ERK-3 are found in TNM stage II and III tumors or Borrmann II tumors. The exact roles of ERK-3 in human cancer carcinogenesis has not been identified. Its upstream and downstream molecular targets are also unidentified. Our data show that ERK-3 is associated with the progression of gastric cancer, but the role is unclear. ERK-3 could be activated in certain morphological subtypes of gastric cancer, and might play an important role in a certain stage. Future investigation will provide the necessary information to shed lights on these points.
The ERK signaling pathway is associated with progression and metastatic potential of tumors. When we analyzed the relationship between ERK-1 and ERK-2 expression and clinicopathological characteristics of the patients, overexpression of these proteins was positively correlated with later TNM staging, lymph node involvement, and serosa invasion in poorly differentiated gastric carcinomas. Lengye et al[23] suggested the role of ERK pathway in regulation of urokinase-type plasminogen activator expression in NIH3T3 cells. Shibata et al[24] found that Ras signaling was required for enhanced matrix metalloproteinase 9 secretions induced by fibronectin in ovarian cancer cells. Taken together, our data show that overexpression of ERK-1 and ERK-2 is part of its upregulation in gastric cancer. Upregulation of ERKs may therefore play an important role in the progression and metastatic potential of gastric cancer.
Several recent studies have demonstrated that tumor cell invasion is associated with p38 MAPK signaling pathway[25,26]. Huang et al[25] showed that endogenous p38 MAPK activity correlated well with breast carcinoma cell invasiveness. They have suggested that p38 MAPK signaling pathway is important for the maintenance of BT549 breast cancer cell invasive phenotype by promoting the stabilities of uPA and uPAR mRNA. In our study, the expression of p38 protein in gastric cancer was also increased. No statistically significant association was found between the IODtumor/IODnormal ratio of p38 protein with age, sex, histological classification, or TNM staging of patients. However in Borrmann IV cancers, there was an increase in p38 expression compared with Borrmann II and III tumors. Our data may shed lights on the possible role of p38 in gastric cancer tumorigenesis. Further work will focus on the p38 activity in gastric cancer and the role of p38 pathway in gastric cancer tumorigenesis and metastasis.
Alterations in the upstream signals could lead to subsequent alterations within the intracellular phosphorylation cascade. Our data show that the protein level of MEK-1 is increased in gastric cancer tissues and consistent with that of ERKs, suggesting that up regulated upstream signals are amplified through intracellular phosphorylation cascade and could lead to constitutive activation of MAPK signaling pathway.
Although the cellular content of metastatic lymph nodes was heterogeneous, we found that in metastatic lymph nodes, the expression of ERK-2, ERK-3, and MEK-1 was increased. Immunohistochemical data also show that the expression of MEK-1 in gastric cancer cells metastasized to lymph nodes is higher than that of the primary sites (Figures 2A-C). Sivaraman et al[27] used in situ RT-PCR to detect the expression of ERK mRNA in breast cancer cells metastasized to involved lymph nodes and found intense staining in the metastatic cancer cells, but not in the surrounding stromal cells within the lymph node. Webb et al[28] also found that the activity of ERK in metastatic NIH3T3 transformed cells was higher than that in parent cells. Their data suggest that overexpression of MAP kinase may potentiate metastases. All in all, upregulation of the MAP kinase pathway might be associated with malignant potential in gastric cancer and can potentiate metastases.
In conclusion, upregulation of ERK pathway rather than p38, plays a more important role in cancer tumorigenesis, aggressiveness and metastases. Future studies are required to better delineate the effectors that mediate the malignant phenotype. Studies, which assess resected human samples, are essential to our better understanding of the signaling mechanisms underlying the regulation of gastric carcinogenesis.
Assistant Editor Guo SY Edited by Wang XL
| 1. | Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2959] [Cited by in RCA: 2761] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 2. | Guo HQ, Guan P, Shi HL, Zhang X, Zhou BS, Yuan Y. Prospective cohort study of comprehensive prevention to gastric cancer. World J Gastroenterol. 2003;9:432-436. [PubMed] |
| 3. | Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 442] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 4. | Jatzko GR, Lisborg PH, Denk H, Klimpfinger M, Stettner HM. A 10-year experience with Japanese-type radical lymph node dissection for gastric cancer outside of Japan. Cancer. 1995;76:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Bremers AJ, Rutgers EJ, van de Velde CJ. Cancer surgery: the last 25 years. Cancer Treat Rev. 1999;25:333-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl). 1996;74:589-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1227] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 8. | Zheng CF, Guan KL. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J Biol Chem. 1993;268:11435-11439. [PubMed] |
| 9. | Lenormand P, Sardet C, Pagès G, L'Allemain G, Brunet A, Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 540] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 506] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 11. | Manni A, Wechter R, Gilmour S, Verderame MF, Mauger D, Demers LM. Ornithine decarboxylase over-expression stimulates mitogen-activated protein kinase and anchorage-independent growth of human breast epithelial cells. Int J Cancer. 1997;70:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, Ahn NG. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1085] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 13. | Salh B, Marotta A, Matthewson C, Ahluwalia M, Flint J, Owen D, Pelech S. Investigation of the Mek-MAP kinase-Rsk pathway in human breast cancer. Anticancer Res. 1999;19:731-740. [PubMed] |
| 14. | Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 291] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Leav I, Galluzzi CM, Ziar J, Stork PJ, Ho SM, Loda M. Mitogen-activated protein kinase and mitogen-activated kinase phosphatase-1 expression in the Noble rat model of sex hormone-induced prostatic dysplasia and carcinoma. Lab Invest. 1996;75:361-370. [PubMed] |
| 16. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 157357] [Article Influence: 3211.4] [Reference Citation Analysis (0)] |
| 17. | Wang S, Evers BM. Caco-2 cell differentiation is associated with a decrease in stat protein levels and binding. J Gastrointest Surg. 1999;3:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Liang B, Wang S, Zhu XG, Yu YX, Cui ZR, Jiang KW, Wang ZZ, Yu YZ. Increased expression of extracellular signal-regulated kinase in human gastric cancer. Zhonghua Putong Waike Zazhi. 2001;16:409-412. |
| 19. | Iwase T, Tanaka M, Suzuki M, Naito Y, Sugimura H, Kino I. Identification of protein-tyrosine kinase genes preferentially expressed in embryo stomach and gastric cancer. Biochem Biophys Res Commun. 1993;194:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Gonzalez FA, Raden DL, Rigby MR, Davis RJ. Heterogeneous expression of four MAP kinase isoforms in human tissues. FEBS Lett. 1992;304:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Sauma S, Friedman E. Increased expression of protein kinase C beta activates ERK3. J Biol Chem. 1996;271:11422-11426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Wang Q, Ding Q, Dong Z, Ehlers RA, Evers BM. Downregulation of mitogen-activated protein kinases in human colon cancers. Anticancer Res. 2000;20:75-83. [PubMed] |
| 23. | Lengye E, Singh B, Gum R, Nerlov C, Sabichi A, Birrer M, Boyd D. Regulation of urokinase-type plasminogen activator expression by the v-mos oncogene. Oncogene. 1995;11:2639-2648. [PubMed] |
| 24. | Shibata K, Kikkawa F, Nawa A, Thant AA, Naruse K, Mizutani S, Hamaguchi M. Both focal adhesion kinase and c-Ras are required for the enhanced matrix metalloproteinase 9 secretion by fibronectin in ovarian cancer cells. Cancer Res. 1998;58:900-903. [PubMed] |
| 25. | Huang S, New L, Pan Z, Han J, Nemerow GR. Urokinase plasminogen activator/urokinase-specific surface receptor expression and matrix invasion by breast cancer cells requires constitutive p38alpha mitogen-activated protein kinase activity. J Biol Chem. 2000;275:12266-12272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Miki H, Yamada H, Mitamura K. Involvement of p38 MAP kinase in apoptotic and proliferative alteration in human colorectal cancers. Anticancer Res. 1999;19:5283-5291. [PubMed] |
| 27. | Sivaraman VS, Wang H, Nuovo GJ, Malbon CC. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest. 1997;99:1478-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 339] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Webb CP, Van Aelst L, Wigler MH, Vande Woude GF. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci USA. 1998;95:8773-8778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |