Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7560
Revised: April 23, 2005
Accepted: April 30, 2005
Published online: December 28, 2005
AIM: To evaluate the safety and efficacy of chronic administration of losartan on hepatic fibrosis in chronic hepatitis C patients.
METHODS: Fourteen patients with chronic hepatitis C non-responders (n = 10), with contraindications (n = 2) or lack of compliance (n = 2) to interferon plus ribavirin therapy and liver fibrosis were enrolled. Liver and renal function test, clinical evaluation, and liver biopsies were performed at baseline and after losartan administration at a dose of 50 mg/d during the 6 mo. The control group composed of nine patients with the same inclusion criteria and paired liver biopsies (interval 6-14 mo). Histological activity index (HAI) with fibrosis stage was assessed under blind conditions by means of Ishak’s score. Subendothelial fibrosis was evaluated by digital image analyses.
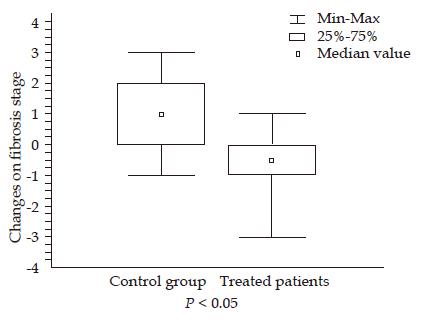
RESULTS: The changes in the fibrosis stage were significantly different between losartan group (decrease of 0.5±1.3) and controls (increase of 0.89±1.27; P<0.03). In the treated patients, a decrease in fibrosis stage was observed in 7/14 patients vs 1/9 control patients (P<0.04). A decrease in sub-endothelial fibrosis was observed in the losartan group. No differences were found in HAI after losartan administration. Acute and chronic decreases in systolic arterial pressures (P<0.05) were observed after the losartan administration, without changes in mean arterial pressure or renal function.
CONCLUSION: Chronic AT-II type 1 receptor (AT1R) blockade may reduce liver fibrosis in patients with chronic hepatitis C.
- Citation: Sookoian S, Fernández MA, Castaño G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: A pilot study. World J Gastroenterol 2005; 11(48): 7560-7563
- URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7560.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7560
The hepatitis C virus (HCV) is one of the leading causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Although major progress has been made in the treatment of chronic HCV infection, the current treatment regimens, including Peginterferons in association with ribavirin, appear capable of eradicating HCV in only 30-50% of the treated patients[1]. In this scenario, alternative medical strategies to reduce hepatic fibrosis are under investigation, since drugs with antifibrotic effects may be an option in the treatment of patients with chronic hepatitis C who do not respond to the standard antiviral therapy.
Much evidence suggests that hepatic stellate cells (HSCs) play important roles in the pathogenesis of liver fibrosis, since they were shown to undergo a transformation during the injury that is termed as activation[2]. Furthermore, in vitro studies have shown that angiotensin II (ANGII) is a mitogenic protein for a number of cell types and between them the HSCs undergo a MAPK-dependent pathway. In these cells, ANGII upregulates the transforming growth factor beta 1 expression via AT-II type 1 receptor (AT1R) in vitro. Additionally, proliferation of activated HSCs was found in chronic liver diseases taking part in the development of liver fibrosis[3]. The fact that the actions of ANGII are mediated through AT1R is related to the therapeutic interventions, since AT1R can be completely blocked by losartan, a specific ANGII receptor antagonist. For instance, ANGII stimulated mRNA expression of TGF-beta and fibronectin can be reversed by saralasin and losartan, a nonselective and specific antagonists for AT1R receptors, respectively[4]. Lastly, in animal models of fibrosis, chronic administration of losartan prevented the development of hepatic fibrosis and portal hypertension[3,5,6].
The purpose of this study was to investigate the safety and efficacy of chronic administration of losartan on hepatic fibrosis in chronic hepatitis C patients.
Fourteen outpatients (10 men and 4 women) aged 49.6±13 years, with both chronic hepatitis C infection and biopsy proven liver fibrosis, were enrolled in a pilot study.
Ten patients were previous non-responders to a 12-mo combined therapy of interferon and ribavirin, two had contraindications and two showed lack of compliance to the mentioned treatment.
In the control group, nine sex- and age-matched untreated chronic hepatitis C patients with the same inclusion and exclusion criteria who underwent paired liver biopsies (interval 6-14 mo) were included. No patient had a previous history of autoimmune disease, alcohol intake, current intravenous drug use or other chronic liver diseases. All were negative for hepatitis B surface antigen and anti-human immunodeficiency virus. Their clinical characteristics are shown in Table 1.
| Treated group (n = 14) | Untreated group (n = 9) | |||
| Baseline | Post losartan | Baseline | Second biopsy | |
| Females/males | 4/14 | 1/8 | ||
| Age (yr) | 49.6±13 | |||
| Hematocrit | 45.2±3.59 | 44.1±3.85 | 48.8±4.66 | 47.8±4.15 |
| White blood cells (K/mm3) | 6.66±1.41 | 6.71±1.67 | 5.98±0.95 | 5.78±1.19 |
| Platelets (K/mm3 | 227±80.8 | 246±90.7b | 223±68 | 209±78.4 |
| Glucose (g/L) | 0.99±0.38 | 1±0.29 | 0.95±0.1 | 1.01±0.06 |
| Blood urea nitrogen (g/L) | 0.31±0.06 | 0.32±0.06 | 0.28±0.07 | 0.25±0.04 |
| Creatinine (mg/dL) | 0.88±0.07 | 0.95±0.11 | 0.99±0.07 | 0.96±0.12 |
| Total bilirubin (mg/dL) | 1±0.53 | 1.08±0.63 | 1.35±0.58 | 1.19±0.58 |
| Alkaline phosphatase (IU/L) | 253±118 | 269±68.9 | 264±93.4 | 349±134 |
| Alanine aminotransferase (IU/L) | 90.5±66 | 91.9±47.6 | 264±167a | 169±124 |
| Aspartate aminotransferase (IU/L) | 65.3±41 | 67±40.9 | 161±97.8a | 130±84.5 |
| Cholesterol (mg/dL) | 157±36.4 | 161±42.6 | 166±38.1 | 169±39.2 |
| Albumin (g/dL) | 4.12±0.32 | 4.43±0.24b | 4.32±0.56 | 4.32±0.5 |
| Globulins (g/dL) | 3.64±0.53 | 3.77±0.45 | 3.74±0.63 | 3.91±0.63 |
| G glutamyl transpeptidase (IU/ L) | 63.2±47.9 | 74.5±53.7 | 194±178a | 217±214 |
| Prothrombin time (%) | 103±19.3 | 106±17.4 | 107±8.19 | 97±9.83 |
| KPTT (s) | 32.6±3.38 | 39.6±18.7 | 37.8±6.99 | 34.5±2.38 |
Written informed consent was obtained from all patients, and the local ethical committee approved the study protocol, based on the 1975 Declaration of Helsinki.
Systolic, diastolic, and mean arterial blood pressures were taken by an automatic sphygmomanometer (VR 12, Electronics for Medicine). Liver and renal function tests and liver biopsies were performed at baseline and after the losartan administration at a dose of 50 mg/d during 6 mo (Losacor, kindly donated by Roemmers, Buenos Aires, Argentina). During the losartan administration, patients did not receive any other medication. Clinical and biochemical follow-ups were assessed and blood pressure was monitored during the first hours, at 1 wk and monthly after the losartan administration.
Ultrasound-assisted liver biopsy was performed using modified Menghini needle of 1.4 mm diameter (Hepafix, Braun, Germany). Liver specimens, previously fixed in formalin, were stained with hematoxylin and eosin, Masson trichrome, silver impregnation for reticular fibers, Perls blue and Prussian blue, and examined by a liver pathologist who was blinded to the clinical data of the patients. Histological activity index (HAI) and fibrosis stage were assessed by means of Ishak’s score.
Selected areas for fibrosis quantification included the hepatic lobule but without the portal, periportal, septal, and pericentral vein areas. Histological slides stained for reticular fibers were evaluated for sub-endothelial deposit of types III collagen[7] by a digital image analysis. A previous study of our group using this method showed a high correlation between image analysis and fibrosis stage[8]. Images were obtained using a video camera (Sony CCD Iris camera) attached to a light microscope and converted to digital format on a computer by CAP VIEW Leadtek Winfast 2000 PAL device (Leadtek Research Inc.). Standardization of the images was performed with software Adobe Photoshop 7.0 (Adobe Systems Inc.). The quantification of the selected color areas of the digitalized, standardized, and processed images was performed in a 256 gray tones scale with software Scion image for Windows (2000 Scion Corporation), and expressed in pixels/inch2.
Results were expressed as mean±SD. Differences between proportions were analyzed by χ2 test and the Fisher’s exact test. Differences between the groups were analyzed by paired and unpaired Student’s t- test, by Mann–Whitney U or Wilcoxon matched paired tests, according to the distribution and the scale of measurement. Spearman’s ρ coefficient was utilized for correlations. Significance was established at P<0.05.
The changes in fibrosis stage were significantly different between losartan group and controls (a decrease of 0.64±1.3 vs an increase of 0.89±1.27, respectively; P<0.02) (Table 2). In the treated patients, a decrease in fibrosis stage was observed in 7/14 patients vs 1/9 control patients (P<0.04). Three of the seven patients showed a reduction of two or more points in fibrosis stage. No differences were observed in HAI after losartan administration. Sub-endothelial fibrosis evaluated by image analysis of lobular areas was significantly reduced after losartan administration, without changes in the control group (Table 2 and Figure 1). A significant correlation was found between fibrosis stage and sub-endothelial fibrosis (Spearman’s ρ 0.36, P<0.03). A significant increase was observed in albumin level and platelet counts in the treated patients.
| Treated group (n = 14) | Untreated group (n = 9) | |||
| Baseline | Post losartan | Baseline | Second biopsy | |
| Histology activity index | 8.08±2.56 | 8.54±1.51 | 7.0±2.12 | 7.6±2.3 |
| Fibrosis stage | 3.29±1.44 | 2.64±1.22 | 2.67±2 | 3.56±2.13 |
| Sub-endothelial fibrosis (pixels/inch2) | 2.48±1.04 | 1.00±0.53a | 1.65±0.43 | 1.55±0.27 |
Additionally, no significant differences were found in any clinical or biochemical parameters between responders and non-responders to losartan, except that patients who responded to losartan had higher baseline serum alkaline phosphatase than non-responders (309±132 IU/L vs 194±25 IU/L, P = 0.009).
Acute (3 h) and chronic (1 mo) decreases in systolic arterial pressures in supine position, and a loss of physiologic increase in diastolic pressures from supine to sitting position were observed after losartan administration (Tables 3 and 4). Nevertheless, only one treated patient had a single episode of mild orthostatic hypotension. No differences were observed in renal function tests after losartan administration.
| Supineposition | Sittingposition | |||
| Baseline (10:00 am) | ||||
| Systolic arterial pressure (mmHg) | 132.7±22.1 | 132.2±16.8 | ||
| Diastolic arterial pressure (mmHg) | 80.7±13.4 | 87.8±8.93 | (P<0.0001) | |
| Mean arterial pressure (mmHg) | 98±15.7 | 102.6±11.1 | (P<0.01) | |
| Three hours post losartan | ||||
| Systolic arterial pressure (mmHg) | 124. 7±17.4 | 127.3±14.9 | ||
| Diastolic arterial pressure (mmHg) | 81.1±14.2 | 83.5±11.1 | ||
| Mean arterial pressure (mmHg) | 95. 7±14.8 | 98.1±12 | ||
It is well known that, at present, the most effective therapy for treating hepatic fibrosis is the removal of the causative agent. In chronic hepatitis C, this goal may be achieved with standard antiviral therapy in a great proportion of patients. Nevertheless, non-responder patients may obtain a benefit from the emerging antifibrotic therapies.
Since renin-angiotensin system blockers are promising drugs in reducing the accumulation of scar tissue in experimental models of chronic liver injury, we performed a pilot study with chronic losartan administration in 14 chronic HCV non-responder patients or with contraindications to interferon plus ribavirin regimen.
Liver biopsy is considered the gold-standard method for the assessment of liver fibrosis[9]. In our study, paired biopsy specimens taken at baseline and at the study completion were compared by means of the standard Ishak score and by an image analysis method for quantification of liver fibrosis. Histopathological scores showed that losartan had an inhibitory effect on the progression of hepatic fibrosis stage. Subendothelial fibrosis evaluated by image analysis of lobular areas was significantly decreased after losartan administration in comparison with the untreated control group.
Regarding biochemical evaluation of the patients, liver and renal function tests, and blood cells content did not change in either group, except for the platelet content and the albumin concentration in the losartan treated patients, who evidenced a significant improvement in comparison with the control group. Additionally, serum HCV-RNA levels had no change after the study in both groups (data not shown).
Several authors, including us, demonstrated in randomized controlled trials that losartan had equal or greater effects in lowering portal pressure than propranolol, without serious adverse effects[10-13]. The effects of losartan on portal pressure may be explained by the effect of the blockade of AT1R on the contractility of hepatic activated stellate cells, by means of a decrease in intrahepatic vascular resistance. In addition to these events, antifibrotic effects of AT1 blockade may have actions on the structural component of portal hypertension.
With regard to the safety of this treatment, no serious side effects were noted during the study. Nonetheless, an overall decrease was observed in systolic arterial pressures in supine position.
The pathogenesis of HCV-induced liver fibrosis is poorly understood due to the lack of a rodent model of persistent HCV infection. However, since liver fibrosis is the excessive accumulation of extracellular matrix proteins including collagen, it is rational to assume that any chronic damage of the liver may lead to the same final outcome.
Experimental and human studies have consistently shown that ANGII is involved in the development of fibrosis in cardiac and renal tissues in conditions associated with chronic inflammation. Besides, in vivo studies have shown that ACE inhibitors and AT1 receptors antagonists can limit the progression of cardiac, renal and pulmonary fibrosis.
In human beings, their efficacy has been tested in a few preliminary pilot studies in patients with NASH[14] and in chronic HCV infection[15], suggesting that renin–angiotensin blocking agents may have beneficial effects on fibrosis progression.
In conclusion, this study shows that administration of an AT1R antagonist may improve liver scores of fibrosis stage in patients with chronic hepatitis C, suggesting that losartan may provide an effective new approach to the treatment of non-responders to antiviral therapy. However, a randomized, controlled trial seems to be necessary in order to confirm the benefit of AT1R blocking for hepatic fibrosis in chronic HCV infection. Lastly, one may speculate that the combination treatment of the clinically used Peginterferons and AT1R blocker may provide a new strategy for anti-liver fibrosis treatment in HCV.
Science Editor Ma JY and Guo SY Language Editor Elsevier HK
| 1. | NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46. [PubMed] |
| 2. | Bataller R, Sancho-Bru P, Ginès P, Lora JM, Al-Garawi A, Solé M, Colmenero J, Nicolás JM, Jiménez W, Weich N. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology. 2001;34:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Leung PS, Suen PM, Ip SP, Yip CK, Chen G, Lai PB. Expression and localization of AT1 receptors in hepatic Kupffer cells: its potential role in regulating a fibrogenic response. Regul Pept. 2003;116:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Croquet V, Moal F, Veal N, Wang J, Oberti F, Roux J, Vuillemin E, Gallois Y, Douay O, Chappard D. Hemodynamic and antifibrotic effects of losartan in rats with liver fibrosis and/or portal hypertension. J Hepatol. 2002;37:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Yoshiji H, Noguchi R, Kuriyama S, Yoshii J, Ikenaka Y. Combination of interferon and angiotensin-converting enzyme inhibitor, perindopril, suppresses liver carcinogenesis and angiogenesis in mice. Oncol Rep. 2005;13:491-495. [PubMed] |
| 7. | Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol. 2002;65:109-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 311] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Fernadez A, Castano G, Sookoian S, Lemberg A, Amante M, Parisi C, Perazzo J. A new image analysis method for quantification of liver fibrosis. J Hepatol. 2003;38:216-Abs. [DOI] [Full Text] |
| 9. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 10. | Schneider AW, Kalk JF, Klein CP. Effect of losartan, an angiotensin II receptor antagonist, on portal pressure in cirrhosis. Hepatology. 1999;29:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Castaño G, Viudez P, Frider B, Sookoian S. Discussion on randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology. 2002;122:1544-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Sookoian S, Castaño G, García SI, Viudez P, González C, Pirola CJ. A1166C angiotensin II type 1 receptor gene polymorphism may predict hemodynamic response to losartan in patients with cirrhosis and portal hypertension. Am J Gastroenterol. 2005;100:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | De BK, Bandyopadhyay K, Das TK, Das D, Biswas PK, Majumdar D, Mandal SK, Ray S, Dasgupta S. Portal pressure response to losartan compared with propranolol in patients with cirrhosis. Am J Gastroenterol. 2003;98:1371-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 15. | Terui Y, Saito T, Watanabe H, Togashi H, Kawata S, Kamada Y, Sakuta S. Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology. 2002;36:1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |