Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7555
Revised: July 5, 2005
Accepted: July 12, 2005
Published online: December 28, 2005
AIM: To examine the effect of pseudolaric acid B on the growth of human gastric cancer cell line, AGS, and its possible mechanism of action.
METHODS: Growth inhibition by pseudolaric acid B was analyzed using MTT assay. Apoptotic cells were detected using Hoechst 33258 staining, and confirmed by DNA fragmentation analysis. Western blot was used to detect the expression of apoptosis-regulated gene Bcl-2, caspase 3, and cleavage of poly (ADP-ribose) polymerase-1 (PARP-1).
RESULTS: Pseudolaric acid B inhibited the growth of AGS cells in a time- and dose-dependent manner by arresting the cells at G2/M phase, which was accompanied with a decrease in the levels of cdc2. AGS cells treated with pseudolaric acid B showed typical characteristics of apoptosis including chromatin condensation and DNA fragmentation. Moreover, treatment of AGS cells with pseudolaric acid B was also associated with decreased levels of the anti-apoptotic protein Bcl-2, activation of caspase-3, and proteolytic cleavage of PARP-1.
CONCLUSION: Pseudolaric acid B can dramatically suppress the AGS cell growth by inducing apoptosis after G2/M phase arrest. These findings are consistent with the possibility that G2/M phase arrest is mediated by the down-regulation of cdc2 levels. The data also suggest that pseudolaric acid B can trigger apoptosis by decreasing Bcl-2 levels and activating caspase-3 protease.
- Citation: Li KS, Gu XF, Li P, Zhang Y, Zhao YS, Yao ZJ, Qu NQ, Wang BY. Effect of pseudolaric acid B on gastric cancer cells: Inhibition of proliferation and induction of apoptosis. World J Gastroenterol 2005; 11(48): 7555-7559
- URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7555.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7555
Extracts from the root and trunk barks of the Chinese tree Pseudolarix kaempferi containing pseudolaric acids are used in traditional Chinese medicine for the treatment of fungal infections[1]. Pseudolaric acid B is the major cell-permeable constituent that displays potent antifungal, antifertil, and anti-angiogenic properties[2-7]. Pseudolaric acid B has cancer chemopreventive activity and inhibits in vitro growth of a number of human cancer cell lines, including KB, A-549, HCT-8, P-388, and L-1210 tumor cells[8]. However, no information is at present available on the chemopreventive potentials of pseudolaric acid B on gastric carcinoma.
Apoptosis, a mode of cell death, is a physiologic event that regulates cell number and eliminates damaged cells. Recent studies implicated that apoptosis is a common mechanism through by which chemotherapeutic agents exert their cytotoxicity and that the efficiency of anti-tumor agents is related to the intrinsic propensity of target tumor cells to respond to these agents by apoptosis[9]. In vitro studies have shown that pseudolaric acid B treatment can induce apoptosis in human HeLa cells via the activation of c-Jun N-terminal kinase and caspase-3[10]. In order to exploit the chemotherapeutic potentials of pseudolaric acid B on gastric carcinoma, we treated human gastric cancer cell line, AGS, with pseudolaric acid B to examine its antiproliferative effect and apoptosis-inducing activity. Our results suggest that pseudolaric acid B can inhibit the growth of AGS human gastric cancer cells by inducing cell cycle arrest, which correlates with a marked decrease in the expression of key G2/M-regulating proteins cdc2. The data also suggest that pseudolaric acid B can trigger apoptosis by decreasing Bcl-2 levels and activating caspase-3 protease. Pseudolaric acid B may be used as an effective chemotherapeutic agent against gastric carcinoma.
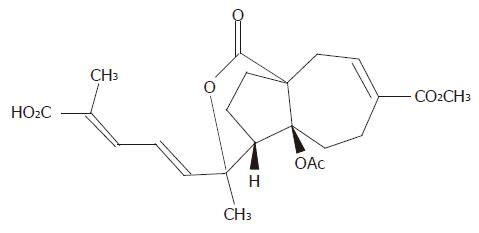
Gastric adenocarcinoma cell line (AGS) was obtained from the Shanghai Institute of Cancer Research. Pseudolaric acid B was purchased from Calbiochem Company (La Jolla, CA, USA). The chemical structure of pseudolaric acid B is shown in Figure 1. Hoechst 33258, RNase A, proteinase K and MTT [3’-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] were purchased from Sigma Chemical Company (St. Louis, USA). Monoclonal antibodies to Cdc2, Bcl-2, caspase-3, and PARP were purchased from Santa Cruz Biotechnology Incorporation (Santa Cruz, CA, USA). PVDF membrane was obtained from Bio-Rad (CA, USA).
AGS was maintained in RPMI 1640 supplemented with 10% fetal calf serum, penicillin G (100 kU/L) and kanamycin (0.1 g/L) in a humidified atmosphere of 95% air and 50 mL/L CO2 at 37 °C. The medium was changed twice a week. A 10 mmol/L stock solution of pseudolaric acid B was prepared in DMSO and stored at -20 °C. Final concentrations of pseudolaric acid B used for different experiments were prepared by diluting the stock with DMEM.
AGS cells were subcultured in a 96-well plate with 1×104 cells/well in 100 µL medium. After 24-h incubation at 37 °C, the medium in each well was discarded and replaced with a fresh medium at various concentrations of pseudolaric acid B in a final volume of 200 µL. Cells were incubated at 37 °C for 6, 12, 24, and 48 h, respectively. At the end of incubation, 50 µL of PBS solution containing 1 mg/mL MTT was added to each well, and further incubated for 4 h. The cell suspension was then centrifuged at 720 r/min for 5 min, and the formazan precipitate in each well was dissolved in 100 µL DMSO for optical density reading at 570 nm.
Control and pseudolaric acid B-treated cells were harvested by trypsinization (0.5% trypsin/2.6 mmol/L EDTA), washed twice with ice-cold PBS and fixed in methanol/PBS (9/1, v/v) at 22 °C for at least 30 min. The fixed cells were then washed twice with ice-cold PBS and stained with 50 mg/mL of propidium iodide in the presence of 25 mg/mL of RNase A. Cell cycle phase distribution was determined by flow cytometry (FACSCalibur, Becton Dickinson) and the data were analyzed by multicycle DNA content and cell cycle analysis software (Modfit LT 2.0).
After being treated with pseudolaric acid B, AGS cells were collected by centrifugation at 1 000 r/min for 5 min and washed twice with PBS. The cells were fixed with 3.7% paraformaldehyde at room temperature for 2 h, centrifuged and washed with PBS, stained with Hoechst 33258 (167 µmol/L) for 30 min at 37 °C. At the end of incubation, the cells were washed and resuspended in PBS for the observation of nuclear morphology under fluorescence microscope (Nikon, Japan).
After being treated with pseudolaric acid B for 12, 24, or 48 h, the cells were harvested and incubated in 0.2 mL lysis buffer containing 10 mmol/L Tris–HCl (pH 8.0), 1 mmol/L EDTA, 1% sodium dodecyl sulfate, and 100 μg/mL proteinase K at 37 °C for 24 h. RNase A (0.5 mg/mL) was added and further incubated at 55 °C for 18 h. Genomic DNA was extracted by phenol/chloroform, precipitated with ethanol and dissolved in TE. Integrity of the DNA was analyzed by electrophoresis on 1.7% agarose gels with ethidium bromide staining.
After being treated with pseudolaric acid B for the indicated periods, the cells were washed with PBS and lysed in a buffer containing 20 mmol/L Tris–HCl, 150 mmol/L NaCl, 1% Triton X-100, 1.5 mmol/L MgCl2, 1 mmol/L NaVO3, 100 mmol/L NaF, 10% glycerol, 1 mmol/L EGTA, 10 mmol/L sodium pyrophosphate, and 1 mmol/L phenylmethylsulfonyl fluoride, pH 7.5. Cell lysates were centrifuged at 12 000 g for 125 min at 4 °C. The protein concentrations were determined using Bio-Rad protein assay (Bio-Rad Laboratories, USA). After SDS-PAGE, proteins were transferred to PVDF membranes for 2 h at 80 mA. Blots were probed with mouse monoclonal antihuman anti-Bcl-2, anti-caspase-3, and rabbit monoclonal anti-human anti-PARP antibodies. Immunoreactivity was detected using either an anti-mouse (Santa Cruz) or an anti-rabbit (Amersham) peroxidase-conjugated secondary immunoglobulin G antibody followed by enhanced chemiluminescence (ECL, Amersham). Experiments were repeated at least thrice.
Data analysis was performed using Student’s t test. P<0.05 was considered statistically significant.
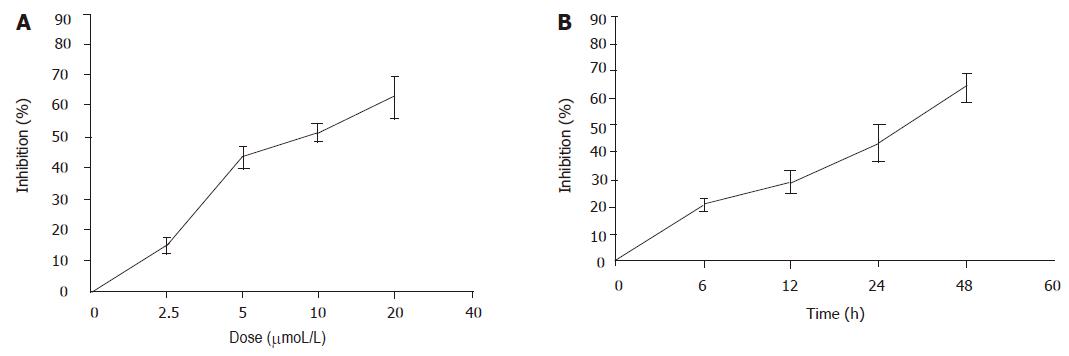
The effects of pseudolaric acid B on cell growth in AGS cell lines were tested. As shown in Figure 2, pseudolaric acid B inhibited AGS cell proliferation in a time- and dose-dependent manner. Only a minor inhibition of AGS cell growth was observed in the presence of 2.5 µmol/L pseudolaric acid B. Growth was inhibited by more than 40% in cells exposed to 5 µmol/L pseudolaric acid B after 24 h. A concentration of 5 µmol/L pseudolaric acid B was used in all further experiments.
We further investigated the effects of pseudolaric acid B on the progression of AGS cells throughout the cell cycle. AGS cells were cultured for various lengths of time in the presence or absence of 5 µmol/L pseudolaric acid B and analyzed by flow cytometry. As shown in Table 1, pseudolaric acid B induced a time-dependent accumulation of AGS cells in the G2/M phase accompanied with a decreased percentage of cells in S and G0/G1 phases of the cell cycle.
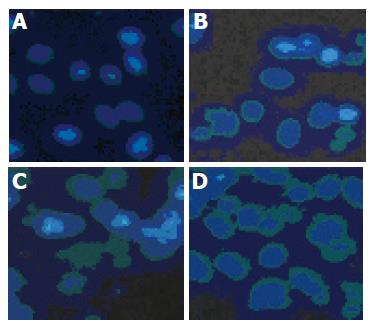
To determine the mode of cell death induced by pseudolaric acid B, morphologic alterations in the AGS cells after treatment with 5 µmol/L pseudolaric acid B for up to 48 h were examined under fluorescent microscope after Hoechst 33342 staining. In the control group, AGS cells were round in shape and stained homogeneously. After 24 h of treatment with pseudolaric acid B, blebbing nuclei and granular apoptotic bodies appeared (Figure 3). We analyzed chromosomal DNA from control and pseudolaric acid B-treated cells. Compared to DNA from control cells, treatment with pseudolaric acid B induced apoptosis, as shown by the formation of distinct internucleosomal DNA fragments (Figure 4). The intensity of the DNA banding ladder progressively increased in a time-dependent manner. An early DNA fragmentation was observed after 12 h of incubation with pseudolaric acid B.
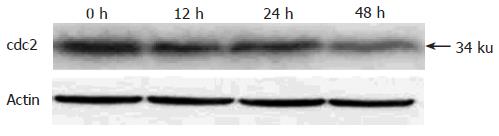
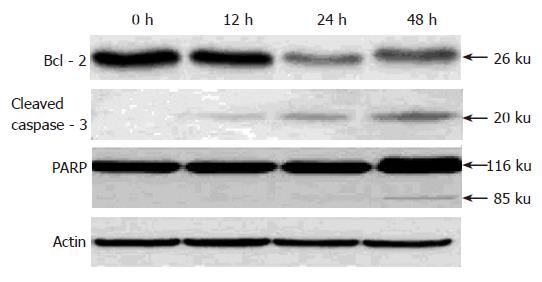
Because pseudolaric acid B arrested AGS cells at the G2/M phase, it was of interest to test the effect of this compound on cdc2 levels, which plays an important role in the G2 to M progression of the cell cycle. A time-dependent reduction in cdc2 protein level was evident in cells treated with pseudolaric acid B for 48 h (Figure 5).
To investigate the mechanism underlying apoptosis induced by pseudolaric acid B, we tested the effect of this compound on Bcl-2 level, an important regulator of apoptotic signaling pathways[19]. As shown in Figure 5, Western blot analysis revealed that pseudolaric acid B treatment decreased Bcl-2 protein levels. We also found that pseudolaric acid B induced proteolytic processing of caspase-3 in a time-dependent manner. Activation of caspase-3 led to the cleavage of a number of proteins, including PARP-1. Pseudolaric acid B treatment also induced a time-dependent proteolytic cleavage of PARP-1, with concomitant accumulation of the 85 ku and the disappearance of the full-size 116 ku molecule (Figure 6). These findings suggested that pseudolaric acid B could induce apoptosis by down-regulating Bcl-2 and activating caspase-3.
Gastric cancer is the second most common cause of cancer death worldwide. Though extensive clinical research has been carried out with numerous combinations of cytotoxic agents, the overall prognosis of advanced gastric cancer still remains poor[11]. Thus, there is considerable interest in identifying more effective cancer chemotherapeutic agents.
Pseudolaric acid B, the diterpenoid isolated from the root and trunk bark of Pseudolarix kaempferi Gordon tree, has been recently reported to exert an antiproliferative effect on several cancer cell lines[10,12,13]. Since there is no report about its effect on human gastric cancer, we treated human gastric cancer cell line, AGS, with pseudolaric acid B to examine its antiproliferative effect and apoptosis-inducting activity. MTT assay showed that pseudolaric acid B could significantly inhibit growth of AGS cells in a dose- and time-dependent manner. In addition, flow cytometric analysis further revealed that pseudolaric acid B induced a time-dependent accumulation of AGS cells in the G2/M phase which was coupled with a parallel depletion of the S and G0/G1 phase. To further analyze the molecular mechanism by which pseudolaric acid B causes cell cycle arrest, we evaluated cdc2 protein levels. The cdc2/cyclin B complex is one of the major regulatory elements governing the G2 to M progression[14]. Cdc2 is activated by phosphorylation of and binding to cyclin B, which is synthesized during the S and G2 phases of the cell cycle[15]. In our study, pseudolaric acid B treatment reduced cdc2 protein levels, suggesting that cell cycle arrest is mediated by the limitation of the supply of cdc2 to cdc2/cyclin B complex formation, which is an essential step in regulating passage into mitosis.
Cell cycle checkpoints are activated to ensure orderly and timely completion of critical events such as DNA replication and chromosome segregation. It is widely accepted that activation of checkpoints in response to DNA damage leads to cell cycle arrest, but in the case of severe damage, the cell cycle arrest leads to cell apoptosis[16,17]. The effects of pseudolaric acid B are compatible with this model. Our present investigation clearly demonstrated that pseudolaric acid B could induce apoptosis of AGS cells, which appears to account for its growth inhibitory and antiproliferative activities. The induction of cell apoptosis was accompanied with characteristic morphological changes, such as chromatin condensation and nuclear fragmentation. Internucleosomal DNA fragmentation as determined by agarose gel electrophoresis also supported the progress of apoptosis of pseudolaric acid B-treated AGS cells.
Apoptosis is a closely regulated process, involving changes in the expression of distinct genes. One of the major genes involved in regulating apoptosis is the protooncogene Bcl-2 encoding a 26-ku mitochondria-associated protein[18,19]. Bcl-2 functions as an intracellular apoptosis suppressor by controlling the mitochondrial membrane permeability with its pro-apoptotic relative, Bax. Diminished expression of Bcl-2 has been observed in certain types of cells undergoing apoptosis[20]. In line with these findings, pseudolaric acid B treatment decreased Bcl-2 protein levels in AGS cells, suggesting that down-regulation of Bcl-2 may be required for AGS cell apoptosis induced by pseudolaric acid B, thus increasing mitochondrial permeability and cytochrome c release, which initiates the progress of apoptosis. In addition, involvement of caspase 3 was also examined in pseudolaric acid B-induced AGS cell apoptosis. Caspase-3, a cysteine protease that exists as an inactive proenzyme in cells, is activated by proteolytic processing at internal aspartic acid residue when cells receive an apoptosis-inducing signal[21]. Activation of caspase-3 leads to degradation and inactivation of key cellular proteins such as DNA repair, signaling, and structural proteins[22,23]. Among these, the cleavage of PARP represents a well described response of cells undergoing caspase-mediated apoptosis[24]. AGS cells treated with pseudolaric acid B exhibited time-dependent activation of caspase-3 and proteolytic cleavage of PARP, indicating that apoptosis of AGS cells is induced by pseudolaric acid B and caspase cascade process.
In conclusion, naturally occurring diterpenoid, pseudolaric acid B, is a potent inhibitor of the growth of human gastric cancer cells. Growth inhibition of the compound is highly related to the induction of cell cycle arrest and apoptosis.
Science Editors Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Li E, Clark AM, Hufford CD. Antifungal evaluation of pseudolaric acid B, a major constituent of Pseudolarix kaempferi. J Nat Prod. 1995;58:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Yang SP, Dong L, Wang Y, Wu Y, Yue JM. Antifungal diterpenoids of Pseudolarix kaempferi, and their structure-activity relationship study. Bioorg Med Chem. 2003;11:4577-4584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Wang WC, Lu RF, Zhao SX, Zhu YZ. [Antifertility effect of pseudolaric acid B]. Zhongguo Yao Li Xue Bao. 1982;3:188-192. [PubMed] |
| 4. | Zhang YL, Lu RZ, Yan AL. [Inhibition of ova fertilizability by pseudolaric acid B in hamster]. Zhongguo Yao Li Xue Bao. 1990;11:60-62. [PubMed] |
| 5. | Wang WC, Gu ZP, Koo A, Chen WS. [Effects of pseudolaric acid B on blood flows of endometrium and myometrium in pregnant rats]. Zhongguo Yao Li Xue Bao. 1991;12:423-425. [PubMed] |
| 6. | Tan WF, Zhang XW, Li MH, Yue JM, Chen Y, Lin LP, Ding J. Pseudolarix acid B inhibits angiogenesis by antagonizing the vascular endothelial growth factor-mediated anti-apoptotic effect. Eur J Pharmacol. 2004;499:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Li MH, Miao ZH, Tan WF, Yue JM, Zhang C, Lin LP, Zhang XW, Ding J. Pseudolaric acid B inhibits angiogenesis and reduces hypoxia-inducible factor 1alpha by promoting proteasome-mediated degradation. Clin Cancer Res. 2004;10:8266-8274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Pan DJ, Li ZL, Hu CQ, Chen K, Chang JJ, Lee KH. The cytotoxic principles of Pseudolarix kaempferi: pseudolaric acid-A and -B and related derivatives. Planta Med. 1990;56:383-385. [PubMed] [DOI] [Full Text] |
| 9. | Kim R, Tanabe K, Uchida Y, Emi M, Inoue H, Toge T. Current status of the molecular mechanisms of anticancer drug-induced apoptosis. The contribution of molecular-level analysis to cancer chemotherapy. Cancer Chemother Pharmacol. 2002;50:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 193] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Gong X, Wang M, Wu Z, Tashiro S, Onodera S, Ikejima T. Pseudolaric acid B induces apoptosis via activation of c-Jun N-terminal kinase and caspase-3 in HeLa cells. Exp Mol Med. 2004;36:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 12. | Gong XF, Wang MW, Tashiro S, Onodera S, Ikejima T. [Pseudolaric acid B induces human melanoma A375-S2 cell apoptosis in vitro]. Zhongguo Zhong Yao Za Zhi. 2005;30:55-57. [PubMed] |
| 13. | Gong XF, Wang MW, Tashiro S, Onodera S, Ikejima T. Pseudolaric acid B induces apoptosis through p53 and Bax/Bcl-2 pathways in human melanoma A375-S2 cells. Arch Pharm Res. 2005;28:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1972] [Cited by in RCA: 2026] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 15. | Fang F, Newport JW. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991;66:731-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 373] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Orren DK, Petersen LN, Bohr VA. Persistent DNA damage inhibits S-phase and G2 progression, and results in apoptosis. Mol Biol Cell. 1997;8:1129-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Smith ML, Fornace AJ. Mammalian DNA damage-inducible genes associated with growth arrest and apoptosis. Mutat Res. 1996;340:109-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3914] [Cited by in RCA: 3938] [Article Influence: 145.9] [Reference Citation Analysis (0)] |
| 19. | Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 346] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4399] [Cited by in RCA: 4503] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 21. | Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1142] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 22. | Wolf BB, Schuler M, Echeverri F, Green DR. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J Biol Chem. 1999;274:30651-30656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 373] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 23. | Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2337] [Cited by in RCA: 2284] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 24. | Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976-3985. [PubMed] |