Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7515
Revised: July 23, 2005
Accepted: July 28, 2005
Published online: December 21, 2005
AIM: To evaluate the efficacy of CT-maximum intensity projection (CT-MIP) in the detection of gastric varices and their inflowing and outflowing vessels in patients with gastric varices scheduled to undergo balloon-occluded retrograde transvenous obliteration (B-RTO).
METHODS: Sixteen patients with endoscopically confirmed gastric varices were included in this study. All patients were evaluated with CT-MIP using three-dimensional reconstructions, before and after B-RTO.
RESULTS: CT-MIP clearly depicted gastric varices in 16 patients (100%), the left gastric vein in 6 (32.5%), the posterior gastric vein in 12 (75.0%), the short gastric veins in 13 (81.3%), gastrorenal shunts in 16 (100%), the hemiazygos vein (HAZV) in 4 (25.0%), the pericardiophrenic vein (PCPV) in 9 (56.3%), and the left inferior phrenic vein in 9 patients (56.3%). Although flow direction itself cannot be determined from CT-MIP, this modality provided clear images of the inflowing and the outflowing vessels. Moreover, in one patient, short gastric veins were not seen on conventional angiographic portography images of the spleen, but were clearly revealed on CT-MIP.
CONCLUSION: We suggest that CT-MIP should be considered as a routine method for detecting and diagnosing collateral veins in patients with gastric varices scheduled for B-RTO. Furthermore, CT-MIP is more useful than endoscopy in verifying the early therapeutic effects of B-RTO.
- Citation: Ishikawa T, Ushiki T, Mizuno KI, Togashi T, Watanabe K, Seki KI, Ohta H, Yoshida T, Takeda K, Kamimura T. CT-maximum intensity projection is a clinically useful modality for the detection of gastric varices. World J Gastroenterol 2005; 11(47): 7515-7519
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7515.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7515
Gastric varices have been increasingly recognized as a major cause of gastrointestinal bleeding in patients with portal hypertension[1,2]. These varices are appreciably less common than their more usual esophageal counterparts, but are associated with a poor prognosis.
Recent technical advances offer us increasingly greater imaging clarity of gastric varices. However, diagnosing this type of varices is problematic, mostly due to the absence of data on the hemodynamic features of such patients. Moreover, a non-invasive imaging modality is preferable in these high-risk patients. CT is currently reported to be superior to angiography in the detection of paraumbilical and retroperitoneal varices[3]. Furthermore, CT-MIP as a rendering method for visualizing the portal system provides superior visualization of portal collaterals.
The purpose of this study was to assess the accuracy and utility of CT-MIP in evaluating the collateral veins in patients with gastric varices before and after B-RTO. We also evaluated the detection of gastric varices and their inflowing and outflowing vessels by CT-MIP.
Sixteen consecutive patients with gastric varices confirmed by endoscopy at the Saiseikai Niigata Second Hospital were enrolled in this study between April 1999 and April 2004. Both CT-MIP and transarterial portography were performed in these patients before and after B-RTO. Patients [8 men and 8 women, aged 45-72 years (mean 59.31 years)] were selected for routine endoscopic screening for esophago-gastric varices. The cause of portal hypertension was liver cirrhosis in 14 patients, and cirrhosis coupled with hepatocellular carcinoma in two patients. Cirrhosis was diagnosed through imaging, and regarding stage, 13 patients were classified as Child-Pugh class B and 3 as Child-Pugh class C. The etiology of liver disease was hepatitis B virus (HBV); in 1 patient (surface antigen (HBs)-positive), hepatitis C virus (HCV) in 7 (anti-HCV antibody-positive), alcoholic liver disease in 5, and autoimmune hepatitis in 3. Endoscopic findings of gastric varices were evaluated according to the general rules proposed by the Japanese Research Society for Portal Hypertension. Variceal form (F) was classified as small and straight (F1, n = 0); enlarged and tortuous (F2, n = 7), or large and coil-shaped (F3, n = 9). Variceal location (L) was classified as: cardiac (Lg-c; located adjacent to the cardiac orifice, n = 0); fundal (Lg-f; located far from the cardiac orifice, n = 11); or cardiofornical (Lg-cf; located between the cardiac orifice and the fornix, n = 5). Patient parameters and characteristics of varices are shown in Table 1.
| Case | Age | Gender | Etiology | GV | Child | HCC |
| 1 | 59 | M | HBV | Lg f F3 | C | + |
| 2 | 69 | F | AIH | Lg cf F2 | B | - |
| 3 | 56 | F | ALC | Lg f F2 | B | - |
| 4 | 69 | F | HCV | Lg f F3 | B | - |
| 5 | 53 | F | AIH | Lg f F3 | B | - |
| 6 | 54 | M | HCV | Lg f F2 | B | + |
| 7 | 64 | M | HCV | Lg cf F3 | B | - |
| 8 | 53 | F | HCV | Lg cf F2 | B | - |
| 9 | 45 | M | ALC | Lg f F3 | B | - |
| 10 | 50 | M | ALC | Lg cf F3 | B | - |
| 11 | 56 | F | ALC | Lg f F3 | B | - |
| 12 | 72 | F | HCV | Lg c F3 | B | - |
| 13 | 65 | M | HCV | Lg c F3 | C | - |
| 14 | 62 | F | AIH | Lg f F2 | B | - |
| 15 | 72 | M | HCV | Lg c F2 | B | - |
| 16 | 50 | M | ALC | Lg cf F2 | C | - |
Follow-up endoscopic examination was performed to evaluate the size of gastric varices at 1 wk, 1 mo, and 3 mo after B-RTO, with CT-MIP also performed at these times together.
All CT studies were performed using an SD-CT scanner (HiSpeed LX/i; General Electric Medical Systems, Milwaukee, WI, USA). Gantry rotation time was 0.8 s. Initially, non-contrast enhanced images were obtained for localization with 10.0 mm beam collimation. Subsequently, after placing a 20-gauge plastic cannula in an antecubital vein, a bolus of 100 mL of iopamidol (Omnipaque; Daiichi Pharmacy Co., Ltd, Tokyo, Japan) was injected with an automated power injector (Auto Enhance A-50; Nemoto Kyorindo, Tokyo) at the rate of 3 mL/s. Scan delays were empirically set at 25, 50, and 200 s after contrast material injection for the arterial, portal, and equilibrium phases, respectively. The entire liver was scanned with a slice thickness of 2.5 mm and table feed of 15 mm (pitch of 6) in a cranio-caudal direction in the arterial phase. In the portal phase, scanning was performed at a slice thickness of 2.5 mm and table feed of 7.5 mm (pitch of 3) in a cranio-caudal direction. In the equilibrium phase, slice thickness was set at 5 mm and table feed at 15 mm (pitch of 3) in a cranio-caudal direction.
Sources with 50% overlap were obtained from the raw data of the portal phase, and a total of 250-350 images were generated. These images were transferred to a workstation [Advantage Workstation 3.1; General Electric Medical Systems, Milwaukee, WI, USA), and three CT portographic models (volume rendering [VR], MIP, and surface shade display (SSD)] were reconstructed. In MIP and VR, voxels over -200 HU were selected. CT portography was reconstructed using the VR algorithm with lower thresholds of 70-90 HU. Display parameters including width, level, opacity, and brightness were chosen subjectively to best visualize the portal vasculature. For MIP, a slab of some 3-10 cm was applied to avoid interference of the vertebral bodies, and the overlying skeletal structures were carefully removed if necessary. Voxel data over the threshold level of 70-90 HU were extracted and applied for SSD images.
Transarterial portography was performed with the digital subtraction angiography technique (DSA, General Electric Medical Systems, Milwaukee, WI, USA) in all the 16 patients to evaluate portosystemic collaterals by the Seldinger method. In all cases, transarterial portography was performed through both the superior mesenteric and splenic arteries using the standard angiographic technique. After the administration of prostaglandin E1 (Palcus, Taisho Pharmacy Co., Ltd, Tokyo), a total of 25 mL of contrast material was injected by power injector at a rate of 5 mL/s via the superior mesenteric artery. Portography via the splenic artery was also obtained with 20 mL of contrast material at a rate of 5 mL/s. Balloon-occluded retrograde transvenous venography (B-RTV) was performed using a DSA device, and was done with selective injection of contrast material into the gastrorenal shunt in all the patients.
After confirming complete visualization of the gastric varices, the sclerotic agent (a mixture of equal amounts of 10% ethanolamine oleate and contrast medium) was injected through the balloon catheter until stasis was obtained in the gastric varices. Eradication of all gastric varices was achieved in all the patients.
All CT-MIP studies were of diagnostic quality, identifying the main portal veins and clearly delineating gastric varices in all the 16 patients (100%). Inflowing vessels to gastric varices were revealed by CT-MIP in all the patients (100%) and comprised the left gastric vein in 6 (32.5%), the posterior gastric vein in 12 (75.0%), and short gastric veins in 13 (81.3%) (Table 2). Regarding outflowing vessels from gastric varices, CT-MIP identified gastrorenal shunts in 16 patients (100%), the hemiazygos vein (HAZV) in 4 (25.0%), the pericardiophrenic vein (PCPV) in 9 (56.3%), and the left inferior phrenic veins in 9 patients (56.3%) (Table 2).
| Case | Inflowing Vessels | Outflowing Vessels | |||||
| LGV | PGV | SGV | G-R shunt | PCPV | HAZV | LIPV | |
| 1 | - | + | - | + | + | - | + |
| 2. | - | - | + | + | - | - | + |
| 3 | - | + | + | + | - | - | + |
| 4 | + | + | + | + | + | - | + |
| 5 | - | + | + | + | - | - | + |
| 6 | - | - | + | + | - | - | - |
| 7 | - | + | + | + | + | - | - |
| 8 | + | + | + | + | - | - | + |
| 9 | - | + | + | + | + | + | - |
| 10 | + | + | + | + | + | + | + |
| 11 | - | + | + | + | + | + | + |
| 12 | - | + | + | + | + | - | - |
| 13 | + | + | + | + | + | + | + |
| 14 | + | - | - | + | - | - | - |
| 15 | + | - | - | + | - | - | - |
| 16 | - | + | + | + | + | - | - |
We then investigated collateral imaging, comparing CT-MIP with angiographic findings (conventional arterial portography) in all the patients. Gastric varices were delineated in all the 16 patients by both CT-MIP and angiography. Both CT-MIP and angiography depicted the gastrorenal shunt in all patients who were studied. Findings of CT-MIP and angiography agreed for the left gastric vein in six patients, for the short gastric vein in one, for the left gastric vein and short gastric vein in one, and for the left gastric vein and the posterior gastric vein in one. In two patients, CT-MIP revealed the short gastric vein as an inflowing vessel to gastric varices, while angiography failed to identify these veins (Table 3). However, CT-MIP failed to reveal the outflowing blood vessels in two patients, while angiography identified the left inferior phrenic vein and PCPV as outflowing vessels in these cases (Table 4). No statistically significant difference between CT-MIP and conventional angiography was apparent in the detection rates of gastric varices and their inflowing/outflowing vessels.
| Inflowing vessels | CT-MIP imaging | Conventional DSA |
| LGV | 6 | 6 |
| PGV | 12 | 12 |
| SGV | 13 | 11 |
| Outflowing vessels | CT-MIP imaging | Conventional DSA |
| G-R shunt | 16 | 16 |
| PCPV | 8 | 9 |
| LIPV | 8 | 9 |
| HAZV | 4 | 4 |
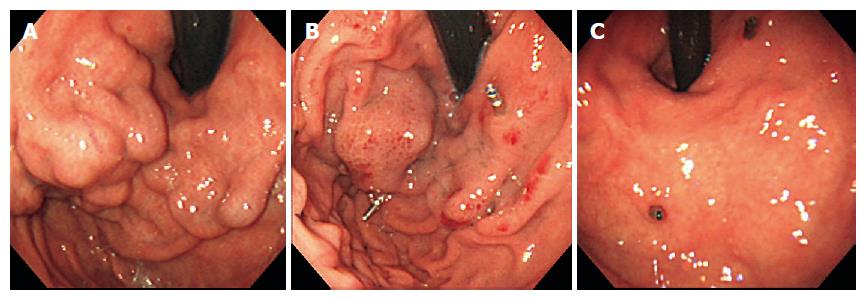
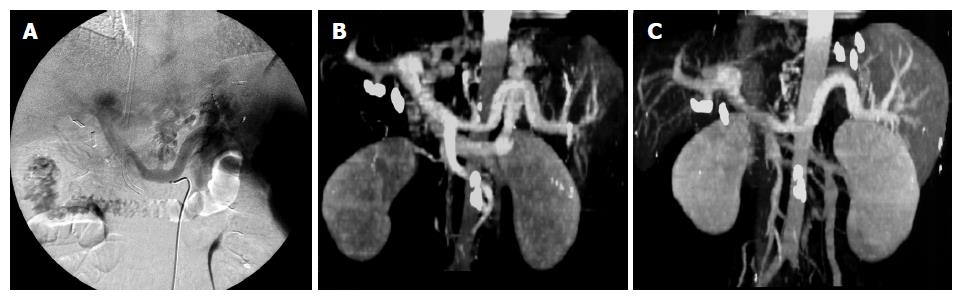
In case 5, in a 53-year-old woman, the endoscopic view revealed Lg-f, F3 type varices (Figure 1A). Using CT-MIP, clear images of gastric varices were obtained and agreed with those of angiographic findings (Figures 2A and B). The short gastric vein and the posterior gastric vein were identified as inflowing, and the gastrorenal shunt and left inferior phrenic vein as outflowing. One week after the procedure, endoscopy revealed unchanged variceal size, although the gastric varices had developed bronze discoloration (Figure 1B). One month after B-RTO therapy, complete disappearance of the varices was observed and this persisted for about 3 mo (Figure 1C).
CT-MIP at 1 wk after the treatment revealed disa-ppearance of the gastrorenal shunt (Figure 2C). CT-MIP was therefore superior to conventional angiography in evaluating treatment of gastric varices in all cases.
The increasing numbers of patients being treated for portal hypertension has led to a growing demand for radiographic examination of the portal venous system. Several methods including endoscopy and intravascular interventional techniques such as balloon-occluded retrograde transvenous obliteration (B-RTO) are available for treating esophageal and gastric varices[4]. Evaluation of the portosystemic collateral pathways in patients with portal hypertension is essential and potentially helpful for clinical management.
For many years, transcatheter portography via the superior mesenteric or splenic arteries has been considered as a standard technique for visualizing the portal vein. However, it is gradually being challenged by non-invasive means of portal venous assessment. Several reports have demonstrated the usefulness of CT for examining the patency of the portal venous system for assessing portosystemic collaterals and evaluating the presence and patency of portosystemic shunts[5,6]. Studies have shown the clinical usefulness of CT-MIP in various diagnostic fields. Particularly in assessing intracranial vessels, CT-MIP has become a non-invasive alternative to conventional angiography for depicting aneurysms and arteriovenous malformations[7]. In contrast, CT-MIP has seldom been used in evaluating abdominal veins, particularly portosystemic collaterals. Recently, however, CT-MIP has been reported to be useful for evaluating the portal venous system in patients with portal hypertension[8] and it is possible that this modality is more sensitive than conventional angiography in detecting esophageal and gastric varices. To the best of our knowledge, no attempt has been made to evaluate whether CT-MIP might yield useful information on gastric varices, and only a few reports have compared the results of CT-MIP with those of conventional angiographic portography regarding visualization of portosystemic collaterals[9].
We compared CT-MIP for portal venous phase images and DSA. We observed the hemodynamics of the gastric varices before and after B-RTO by means of CT-MIP. The inflowing vessels to gastric varices generally consist of the short gastric veins, the posterior gastric vein, and the left gastric vein, while the outflowing vessels are commonly gastrorenal shunts. In this study, CT-MIP more thoroughly delineated gastrorenal shunts, the left gastric vein, and gastric varices than did angiography and CT. Unlike DSA, CT-MIP is unable to demonstrate direction of the flow within the portal venous system. However, CT-MIP proved so effective that it can be considered as a less invasive alternative to conventional angiographic portography for assessing portosystemic collaterals.
CT-MIP was associated with the following limitations. Small vessels were not defined as well with CT-MIP as with retrograde venography performed during balloon occlusion. The modality is also prone to certain artifacts, such as those due to surrounding tissue and marking clips. In addition, it was difficult to distinguish between intramural gastric varices and extramural collateral veins using CT-MIP.
On the other hand CT-MIP has certain advantages. Firstly, it is a non-invasive modality. Furthermore, CT-MIP is useful for detecting the entire length of gastric varices, which is not always possible with B-RTV due to the limitations of venography. This study has emphasized that CT-MIP may be useful for the treatment of patients with gastric varices[10]. Moreover, the technique may be useful for evaluating the effect of embolization therapy[11]. Endoscopic findings of gastric varices after B-RTO are particularly limited, owing to the depth of submucosal or extramural collateral veins. On endoscopic findings following B-RTO, gastric varices gradually become reduced in size, disappearing completely after some months. Hence, CT-MIP revealed disappearance of gastrorenal shunts and gastric varices after B-RTO.
CT-MIP enabled us to detect the blood flow from gastric varices to other collateral shunts and is therefore useful in the follow-up after therapeutic procedures[12].
We suggest that CT-MIP can be used as a routine method for detecting and diagnosing collateral veins in patients with gastric varices and that it is effective for use with embolization therapy for gastric varices. Moreover, CT-MIP may also be useful for the assessment of therapeutic effect of B-RTO and could potentially demonstrate residual gastric varices after B-RTO, better than endoscopy.
We conclude that CT-MIP is a useful method for the assessment of therapeutic effect following treatment of gastric varices. Although further evaluation and the development of alternative therapies for the treatment of gastric varices are warranted, it would seem that CT-MIP has a valuable role in this situation.
When considering B-RTO for the treatment of patients with gastric varices, CT-MIP is useful for selecting the patients as candidates, for planning the procedure, and finally for evaluating the results. Furthermore, CT-MIP may be useful for detecting collateral vessels by portal hypertension.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 842] [Article Influence: 25.5] [Reference Citation Analysis (42)] |
| 2. | Ide K, Nagasaki Y, Tomimatsu H, Nakashima T, Arakawa M, Kojiro M. Pathomorphologic and angio-architectural studies of oesophagogastric varices. J Gastroenterol Hepatol. 1989;4 Suppl 1:204-207. [PubMed] |
| 3. | McCain AH, Bernardino ME, Sones PJ, Berkman WA, Casarella WJ. Varices from portal hypertension: correlation of CT and angiography. Radiology. 1985;154:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996;167:1317-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Bernardino ME, Steinberg HV, Pearson TC, Gedgaudas-McClees RK, Torres WE, Henderson JM. Shunts for portal hypertension: MR and angiography for determination of patency. Radiology. 1986;158:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Finn JP, Kane RA, Edelman RR, Jenkins RL, Lewis WD, Muller M, Longmaid HE. Imaging of the portal venous system in patients with cirrhosis: MR angiography vs duplex Doppler sonography. AJR Am J Roentgenol. 1993;161:989-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Korogi Y, Takahashi M, Katada K, Ogura Y, Hasuo K, Ochi M, Utsunomiya H, Abe T, Imakita S. Intracranial aneurysms: detection with three-dimensional CT angiography with volume rendering--comparison with conventional angiographic and surgical findings. Radiology. 1999;211:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 111] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Hughes LA, Hartnell GG, Finn JP, Longmaid HE, Volpe J, Wheeler HG, Clouse ME. Time-of-flight MR angiography of the portal venous system: value compared with other imaging procedures. AJR Am J Roentgenol. 1996;166:375-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Horton KM, Fishman EK. Paraumbilical vein in the cirrhotic patient: imaging with 3D CT angiography. Abdom Imaging. 1998;23:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Leyendecker JR, Rivera E, Washburn WK, Johnson SP, Diffin DC, Eason JD. MR angiography of the portal venous system: techniques, interpretation, and clinical applications. Radiographics. 1997;17:1425-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Ono N, Toyonaga A, Nishimura H, Hayabuchi N, Tanikawa K. Evaluation of magnetic resonance angiography on portosystemic collaterals in cirrhotic patients. Am J Gastroenterol. 1997;92:1515-1519. [PubMed] |
| 12. | Pieters PC, Miller WJ, DeMeo JH. Evaluation of the portal venous system: complementary roles of invasive and noninvasive imaging strategies. Radiographics. 1997;17:879-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |