Published online Dec 14, 2005. doi: 10.3748/wjg.v11.i46.7368
Revised: April 23, 2005
Accepted: April 26, 2005
Published online: December 14, 2005
AIM: To explore the possibility of repression of chloromycetin (Cm) acyl transferase by using external guided sequence (EGS) in order to converse the clinical E coli isolates from Cm- resistant to Cm- sensitive.
METHODS: EGS directed against chloromycetin acetyl transferase gene (cat) was cloned to vector pEGFP-C1 which contains the kanamycin (Km) resistance gene. The recombinant plasmid pEGFP-C1+EGScat1+cat2 was constructed and the blank vector without EGS fragment was used as control plasmids. By using the CaCl2 transformation method, the recombinant plasmids were introduced into the clinically isolated Cm resistant but Km sensitive E coli strains. Transformants were screened on LB agar plates containing Km. Extraction of plasmids and PCR were applied to identify the positive clones. The growth curve of EGS transformed bacteria cultured in broth with Cm resistance was determined by using spectrophotometer at A600. Drug sensitivity was tested in solid culture containing Cm by using KB method.
RESULTS: Transformation studies were carried out on 16 clinically isolated Cm-resistant (250 μg/mL of Cm) E coli strains by using pEGFP-C1-EGScat1cat2 recombinant plasmid. Transformants were screened on LB-agar plates containing Km after the transformation using EGS. Of the 16 tested strains, 4 strains were transformed successfully. Transformants with EGS plasmid showed growth inhibition when grown in liquid broth culture containing 200 μg/mL of Cm. In drug sensitivity test, these strains were sensitive to Cm on LB-agar plates containing 200 μg/mL of Cm. Extraction of plasmids and PCR amplification showed the existence of EGS plasmids in these four transformed strains. These results indicated that the Cat of the four clinical isolates had been suppressed and the four strains were converted to Cm sensitive ones.
CONCLUSION: The EGS directed against Cat is able to inhibit the expression of Cat, and hence convert Cm-resistant bacteria to Cm-sensitive ones. Thus, the EGS has the capability of converting the phenotype of clinical drug-resistant isolates strains to drug-sensitive ones.
- Citation: Gao MY, Xu CR, Chen R, Liu SG, Feng JN. Chloromycetin resistance of clinically isolated E coli is conversed by using EGS technique to repress the chloromycetin acetyl transferase. World J Gastroenterol 2005; 11(46): 7368-7373
- URL: https://www.wjgnet.com/1007-9327/full/v11/i46/7368.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i46.7368
Drug resistance in pathogenic bacteria is a problem of major clinical importance, which has not been effectively solved yet. The traditional approach to this problem is searching for novel antibiotics. However, this is expensive and time consuming, and the bacteria can quickly develop drug resistance to these novel antibiotics. With advances in gene therapy, ribozyme technology[1,2] appeared following antisense RNA technique[3-6], which though is abstract of the activity of catalysis and cleavage. Then, external guide sequence (EGS) technique[7] was found, with the advantages of both ribozyme and antisense RNA. EGS, a synthetic gene coding for small oligoribonucleotides, is able to form complexes with the mRNA encoded by target gene. The complexes are recognized as substrates of RNase P[8-11]. Then, EGS directs RNase P to cleave and inactivate the target mRNA. Therefore, the EGS technique is a method to block a particular gene by inhibiting the translation of its mRNA[12-14]. In 1997, by designing EGS directed against chloromycetin (Cm) acyl transferase (cat), Sidney Altman successfully applied EGS technique in engineered bacteria to convert the Cm-resistant bacteria into Cm-sensitive ones, and hence paved a new way to solve the drug resistance problem[15]. In this study, we investigated the feasibility of applying EGS technique in converting the phenotype of clinically isolated E coli from Cm-resistant to Cm-sensitive.
The E coli samples were clinically isolated from Affiliated Tongji Hospital of Huazhong University of Science and Technology between 2001 and 2002, from which 16 strains resistant to Cm 250 μg/mL and sensitive to kanamycin (Km) 50 μg/mL were selected as test strains. Plasmid pEGFP-C1 and pKB EGS cat1+cat2 were kindly provided by Dr. Sidney Altman (Yale University, USA). The plasmid pKB EGS cat1+cat2 was constructed according to the method in the literature[15].
Construction of pEGFP-C1-EGS cat1+cat2 recombinant plasmids A 510-bp EGS fragment directed against Cm acetyl transferase gene (cat) was cloned into pEGFP-C1 plasmid containing kanamycin-resistant gene between EcoRI and I sites. The EGS includes: M1RNA promoter, EGScat1, hammerhead sequence (HH1, HH2), EGScat2, HH3, and M1 terminator. The recombinant pEGFP-C1 plasmid containing EGScat1+cat2 was named K3 plasmid, while pEGFP-C1 plasmid that had Km resistance gene but without EGS fragment was named as K plasmid.
Identification of partial phenotypes of clinically isolated E coli: (1) Purification of the test bacteria The clinically isolated bacterial strains were streaked on the LB plates and cultured for 6-8 h. A single colony on the plates was selected for Gram staining and its cellular configuration was checked under the microscope. Other single colony was then taken and diluted to 10-8. The clone grown on plates with 30-300 colonies was taken for tests of Cm drug resistance and Km drug sensitivity again, and then Gram stained for morphology check. These steps were repeated until pure bacterium strains were obtained[16]; (2) Extraction of plasmids of the test bacteria and determination of plasmid incompatibility The methods for plasmid extraction and incompatibility determination were described by Huang[17]; (3) Determination of spontaneous mutation rate of the test bacteria The spontaneous mutation rate of testing bacteria to Km was determined for colonies grown on LB and LB+Km plates by using dilution plate counting method[18]; (4) Determination of logarithmic growth stage of test bacteria Bacterial growth curve were plotted based on bacterial optical density (A600)[19].
Transformation of test bacteria by pEGFP-C1-EGScat1+cat2 recombinant plasmids and identification: (1) Transforming test The competent cells of testing bacteria were prepared by using CaCl2 method, and were transformed respectively with plasmids K3 and K[20]. The transformants that received K3 plasmid were designated as testing bacteria, and those transformed with K plasmid without EGS were used as plasmid controls. The competent cells from original testing bacteria were mock-transformed (without plasmid) and served as strain controls. Competent cells were inoculated on LB plates containing 50 μg/mL of Km after transformation, followed by incubation at 37 °C for 12-16 h; (2) Extraction of plasmids and total DNA Plasmid was extracted by using alkaline cleavage method[17] and total DNA was extracted by using the boiling method[21]. Positive clones were selected and transferred into Eppendorf tubes containing 20 μL sterilized water and boiled for 10 min. The supernatant was collected after centrifugation; (3) PCR amplification The primers were designed specifically for the inserted EGS on the vector. The amplified fragment containing EGScat1 and cat2 was 391 bp. The primer pairs used for PCR amplification were 5’-AGCCTGACCGATGATGTTG-3’ and 5’-TCCTCACGGACTCATC AGAC-3’. The PCR reactions were performed at 94 °C for 1 min, 57 °C for 1 min, and 72 °C for 1.5 min, total 30 cycles. The PCR products were electrophoresed on a 1.5% agarose gel; (4) Cm sensitivity test of the transformants After transformation, the colonies grown on plates and the original colonies were re-inoculated on the LB plates containing 200 μg/mL of Cm and plates containing 50 μg/mL of Km, and incubated at 37 °C for 8-12 h. Then, their growth conditions on these two plates were checked and recorded; (5) Growth determination of transformants in liquid broth The colonies (K3, K plasmid transformants and original bacteria) were separately inoculated into 200 mL of broth culture containing Cm (200 μg/mL) with the inoculating concentration A600 = 0.05, and incubated in an orbital shaker at 37 °C and 200 r/min. A 0.5 mL bacterial suspension was sampled hourly and its A value was determined. Growth curve was plotted according to the constantly collected A values. The transformants were also incubated at the same concentrations into PA bottles at 37 °C and the bacterial growth was observed hourly as well.
Sixty-one Cm-resistant but Km-sensitive E coli strains were clinically isolated. Of them, 16 strains were selected as test strains for their comparatively low simultaneous mutation rate. However, the test strains sensitive to Km were still suitable to present the spontaneous mutation and hence obtain Km resistance after culturing in a big scale. Among them, strains 16, 20, 3 900, and 6 470 showed the lowest mutation rate of Km drug resistance, being 8.85×10-6, 3.36×10-6, 6.57×10-7, and 2.96×10-7, respectively. The data of others were little higher than 10-5.
Plasmids’ profile of the test bacteria The plasmid profile of the test strains showed that each contained 2-3 plasmid bands. Introducing the plasmids extracted from test bacteria into E coli DH5α (no indigenous plasmids) indicated that Cm-resistant gene was located in the plasmids. The existence of resistance plasmids in the test strains may account for the difficulty of introducing EGS plasmid into the cells. Furthermore, the DH5α derivatives transformed with the plasmids extracted from test bacteria were hard to be transformed again with K3 and K. This suggested the plasmid incompatibility between original plasmids and K or K3 plasmids.
Determination of logarithmic phase The bacterial growth curve revealed that the logarithmic growth stage was from 4 to 8 h after being inoculated in the broth. In this study, the fresh culture of test strains was harvested in this period to prepare competitive cells.
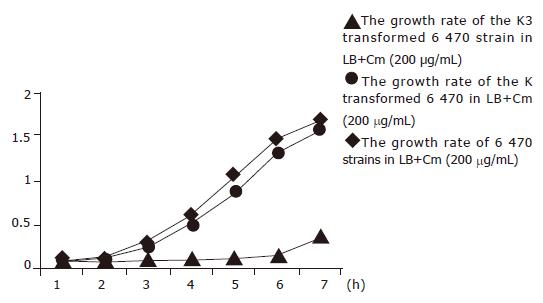
Four strains, 16, 20, 3 900, and 6 470 were transformed with K3 successfully. Kinetic growth study demonstrated that these four strains transformed with K3 plasmid exhibited growth inhibition in the broth containing 200 μg/mL of Cm (Figure 1). The growth inhibition became apparent at 2 h and peaked at 3 and 4 h, but active growth resumed at 5 h. In contrast, neither bacteria transformed with K plasmid nor original bacteria with mock-transformation showed any growth inhibition in the incubation condition described above. Their concentrations increased steadily as demonstrated by A600 values. Although the number of bacteria transformed with EGS began to elevate at 5 h, the degree of growth was much lower than that of the original bacteria. In PA bottles, the K3 plasmid transformed bacterial culture was still clear at 6 h to the naked eye and started to be turbid at 8 h, while the culture for K plasmid transformed bacteria or mock-transformed original bacteria started to be turbid at 3 h and was highly turbid at 6 h. The above findings demonstrated that EGS gene was able to convert bacteria from Cm-resistant to Cm-sensitive phenotype. All of the four strains that obtained their phenotypic conversion of drug resistance showed a lower spontaneous mutation rate, which may contribute to their easy transformation because of their stability. They exhibited very few variant strains when cultured on Km plates, and thus the transformants could be easily identified and collected.
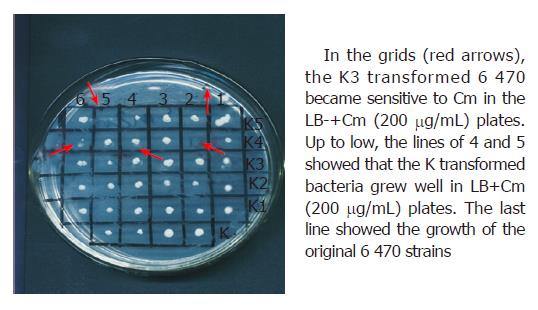
Drug sensitivity test showed that the four strains transformed with K3 plasmid not only obtained sensitivity to Cm in liquid broth culture but also became sensitive to Cm in LB+Cm (200 μg/mL) solid culture (as indicated in Figure 2). The bacteria transformed with K plasmid and the original isolates grew well in LB+Cm (200 μg/mL) solid culture.
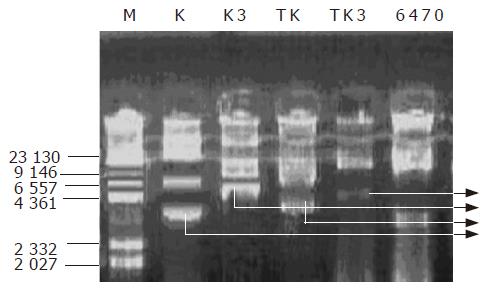
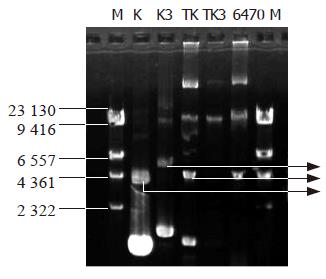
Determination of the plasmids in transformed bacteria Four strains of transformed bacteria were subjected to the determination of the plasmid extraction. Figure 3 is the map of rapid extraction of plasmid and Figure 4 is the map of plasmid extraction after the transformants were cultured for 2 d. Figure 3 shows that the K and K3 can be detected in the strains transformed with K and K3, respectively. This indicated that K3 and K plasmids had been introduced into the cells successfully. In Figure 4, TK had the same bands as K, while TK3 had no similar bands as K3, indicating that the K3 plasmid was lost or had been degraded after 2 d of culturing. This may explain why the transformant retained its growing capacity gradually after several hours of culturing in the broth medium.
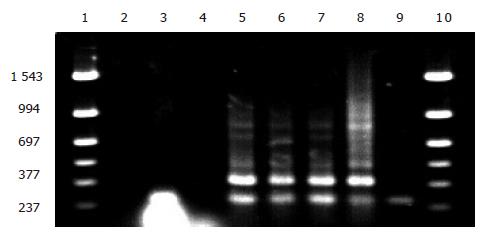
Identification of EGS gene by PCR The colonies transformed with K3, which did not grow on LB+Cm (200 μg/mL) plate (restoration of Cm sensitivity) but grew well in the corresponding LB+Km (50 μg/mL) plate, were used for PCR amplification of EGS fragments. K3 plasmid was used as positive control and clones transformed with K plasmid, original testing bacteria and DNA-free distilled water were used as negative controls. As shown in Figure 5, lanes 5, 6, and 7 were clones transformed with K3 plasmid that did not grow on LB+Cm plate, showing that these clones contained the EGS gene fragment (391 bp, lane 8). Lanes 4 and 9 were original test bacteria and K plasmid transformed bacteria, showing no EGS gene fragment in these strains. Lane 3 was the clone that initially resumed Cm sensitivity but lost the sensitivity 2 d later on LB+Cm plate. PCR amplification failed to detect any EGS band, suggesting the loss of EGS plasmid in this clone.
The problem of microbial drug resistance is growing severely year after year clinically. To cope with this problem, destroying the drug resistance gene is an effective and potential approach, in which no effective method has so far been achieved[22-24]. In 1997, Sidney Altman introduced EGS technique, which conversed the Cm resistance successfully. On the basis of the study on engineered bacteria by Altman, this study further introduced the EGS gene into the clinically isolated bacterial strains. Our study demonstrated EGS-mediated conversion of a Cm-resistant to a Cm-sensitive phenotype, and further showed the successful conversion of drug resistance by the introduction of EGS gene not only in laboratory bacteria but also in clinically isolated bacteria. This indicates a potential clinical utilization.
Mutation is a common phenomenon present in microorganisms. The clinically isolated bacterial strains are characterized with even higher spontaneous mutation rate than lab strains. In the early period of our experiments, it was found that the competent cells of the control bacteria sensitive to Km not transformed with plasmids and originally sensitive to Km still grew on the Km plates. At first, it was mistakenly rendered as the result of impurity of test strain. After further purification and spontaneous mutation study, we understood that it has resulted from high mutation rate of the clinically isolated bacteria. Mutated test strains obtained Km resistance, lost Km-sensitiveness, and therefore made selecting transformants difficult. To solve this problem, our efforts focused on selecting stable strains with low spontaneous mutation rate. At the end of the procedure, 16 comparatively stable strains were screened from the clinically isolated bacterial strains.
In this study, only 4 of 16 test strains were converted from drug-resistant to drug-sensitive phenotype. The low efficiency of conversion may be ascribed to the low transformation efficiency. Firstly, as Enterobacteriaceae, test strains were hard to be transformed for their native low transformation frequency[25]. Secondly, the low transformation rate can be contributed to the existence of host plasmids in test strain which may result in plasmid incompatibility. Thirdly, although the criteria for screening positive transformants in the culture medium containing Km were set up, not all of the transformed bacteria were desired transformants due to the spontaneous mutation of the testing bacteria. We believe that even if we select other sensitive antibiotics to testing bacterial strains, spontaneous mutation would also happen. So suitable methods to screen positive transformants were abstract. Km resistance in this study did not function as an effective selection marker due to the spontaneous mutation of the test strains. To increase the efficiency of conversion, we tried to apply one-step method[26] and electroporation transformation method, but the results were also not satisfactory. Although we can construct recombinant plasmids and insert a fluorescence enzyme’s gene into the vector (EGFP site at pEGFP-C1 vector) to aid in screening, it may increase the transforming difficulty or render the transformed bacteria. In order to reduce the false positive clone in transformation assay, the original bacteria with high spontaneous mutation rate were excluded. At the same time, we found that the efficiency of transformation could be enhanced by using SOC medium and by collecting cell culture at early logarithmic phase. Transformation could be facilitated by washing cell culture with CaCl2 solution for 2-3 times instead of once as well. This might be contributed to the increased permeability of the E coli wall.
PCR amplification was used to identify positive clones. In contrast to the spontaneously mutated bacterial strains, the transformants obtained through transformation grew slowly on the Km plates. On the other hand, long time culturing resulted in the loss of plasmids. Thus, it was difficult to identify the EGS transformants by plasmid extraction. In this study, PCR was applied to identify the presence of EGS in the EGS transformed bacteria and it worked effectively. Certainly, further study is to perform Northern blot to know the attenuation of mRNA transcribed from cat gene, which could further definitely verify the roles of EGS. As our conditions are limited, the experiment was not done.
EGS as a sort of synthesized oligonucleotides can combine the target RNA to form the substrate similar to pre-tRNA, which can be cleaved by RNase P and lose the ability of further gene expression. The targeted mRNA is cleaved but EGS itself is not cleaved[7]. EGS can function continuously. In this study, however, we found that the positive clones regained the Cm resistance after being cultured in broth containing Cm for 8 h and then on solid culture for 2 d. No EGS was detected and no plasmid could be extracted in clones that regained Cm resistance. This demonstrated that EGS plasmid had been lost in the host because of continuous culture. The reason might be due to an incompatibility between indigenous and exogenous plasmids.
Our experiments demonstrated that culturing on Km plates facilitated EGS plasmid inheritance, while culturing on the Cm plates was prone to result in the loss of EGS plasmid. This study also suggested the need for more effective methods to introduce EGS into the bacterial cells and to maintain its stability. Though a lot of challenges were involved in this study, transformants were still obtained. The results reflected the effectiveness of the EGS transforming drug-resistant bacteria, and provided a foundation for the clinical application of EGS.
In recent years, although RNA interference technique has been developed, no RNA interference phenomenon was observed in prokaryotes[27-30]. Thus, the EGS technique still is the most prospective way to solve bacterial drug resistance. As to the development of EGS technique, Sidney Altman put forward that enhancing the promoter, increasing the copy of EGS, augmenting the binding sites of EGS and stabling the EGS-mRNA complex are the potential methods. Furthermore, combined with nanotechnology, the EGS technology could be more promising. Conclusively, to improve this study, more measures should be explored for further investigation.
We are deeply indebted to Professor Altman for kindly providing the plasmid and for his abundant help in this study.
Co-first authors: Mei-Ying Gao and Chuan-Rui Xu
Co-correspondent: Mei-Ying Gao
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Akashi H, Matsumoto S, Taira K. Gene discovery by ribozyme and siRNA libraries. Nat Rev Mol Cell Biol. 2005;6:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Lilley DM. Structure, folding and mechanisms of ribozymes. Curr Opin Struct Biol. 2005;15:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Chan MW, Chan VY, Leung WK, Chan KK, To KF, Sung JJ, Chan FK. Anti-sense trefoil factor family-3 (intestinal trefoil factor) inhibits cell growth and induces chemosensitivity to adriamycin in human gastric cancer cells. Life Sci. 2005;76:2581-2592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Jhaveri MS, Rait AS, Chung KN, Trepel JB, Chang EH. Antisense oligonucleotides targeted to the human alpha folate receptor inhibit breast cancer cell growth and sensitize the cells to doxorubicin treatment. Mol Cancer Ther. 2004;3:1505-1512. [PubMed] |
| 5. | Sarno R, Ha H, Weinsetel N, Tolmasky ME. Inhibition of aminoglycoside 6'-N-acetyltransferase type Ib-mediated amikacin resistance by antisense oligodeoxynucleotides. Antimicrob Agents Chemother. 2003;47:3296-3304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Delihas N, Rokita SE, Zheng P. Natural antisense RNA/target RNA interactions: possible models for antisense oligonucleotide drug design. Nat Biotechnol. 1997;15:751-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Forster AC, Altman S. External guide sequences for an RNA enzyme. Science. 1990;249:783-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 240] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Yuan Y, Altman S. Selection of guide sequences that direct efficient cleavage of mRNA by human ribonuclease P. Science. 1994;263:1269-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Li Y, Guerrier-Takada C, Altman S. Targeted cleavage of mRNA in vitro by RNase P from Escherichia coli. Proc Natl Acad Sci USA. 1992;89:3185-3189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Yuan Y, Hwang ES, Altman S. Targeted cleavage of mRNA by human RNase P. Proc Natl Acad Sci U S A. 1992;89:8006-8010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Werner M, Rosa E, Nordstrom JL, Goldberg AR, George ST. Short oligonucleotides as external guide sequences for site-specific cleavage of RNA molecules with human RNase P. RNA. 1998;4:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Zhang H, Altman S. Inhibition of the expression of the human RNase P protein subunits Rpp21, Rpp25, Rpp29 by external guide sequences (EGSs) and siRNA. J Mol Biol. 2004;342:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Guerrier-Takada C, Li Y, Altman S. Artificial regulation of gene expression in Escherichia coli by RNase P. Proc Natl Acad Sci U S A. 1995;92:11115-11119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Li Y, Altman S. A specific endoribonuclease, RNase P, affects gene expression of polycistronic operon mRNAs. Proc Natl Acad Sci USA. 2003;100:13213-13218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Guerrier-Takada C, Salavati R, Altman S. Phenotypic conversion of drug-resistant bacteria to drug sensitivity. Proc Natl Acad Sci USA. 1997;94:8468-8472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Zhao B, He SJ. Microbiology Lab Manual. First Edition. Beijing: Science press 2002; 121-123. |
| 17. | Huang PT. Molecular Cloning: A Laboratory Manual (Translation). Third Edition. Beijing: Science press 2002; 29-30. |
| 18. | Zhao B, He SJ. Microbiology Lab Manual. First Edition. Beijing: Science press 2002; 187-189. |
| 19. | Zhao B, He SJ. Microbiology Lab Manual. First Edition. Beijing: Science press 2002; 75-76. |
| 20. | Huang PT. Molecular Cloning: A Laboratory Manual(Translation). Third Edition. Beijing: Science press 2002; 96. |
| 21. | Huang PT. Translation. Molecular Cloning: A Laboratory Manual. Third Edition. Beijing: Science press 2002; 41-43. |
| 22. | Tekos A, Stathopoulos C, Tsambaos D, Drainas D. RNase P: a promising molecular target for the development of new drugs. Curr Med Chem. 2004;11:2979-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Altman S. RNA enzyme-directed gene therapy. Proc Natl Acad Sci U S A. 1993;90:10898-10900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Gopalan V, Vioque A, Altman S. RNase P: variations and uses. J Biol Chem. 2002;277:6759-6762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Zheng SL. Basis to Microbiology. First Edition. Beijing: Chemistry industry press 1992; 322-325. |
| 26. | Chung CT, Niemela SL, Miller RH. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172-2175 DOI : 10.1073/pnas.86.7.2172. |
| 27. | Hannon G J. RNAi: A Guide to Gene silencing. First Edition. Photolithograph book. Beijing: Chemistry industry press 2004; 23-24. |
| 28. | Hannon G J. RNAi: A Guide to Gene silencing. First Edition. Photolithograph book. Beijing: Chemistry industry press 2004; 243-246. |
| 29. | Hannon G J. RNAi: A Guide to Gene silencing. First Edition. Photolithograph book. Beijing: Chemistry industry press 2004; 361-364. |
| 30. | Hannon G J. RNAi: A Guide to Gene silencing. First Edition. Photolithograph book. Beijing: Chemistry industry press 2004; 265-267. |