Published online Dec 14, 2005. doi: 10.3748/wjg.v11.i46.7364
Revised: July 21, 2005
Accepted: July 21, 2005
Published online: December 14, 2005
AIM: To detect the platelet-activating factor (PAF) and the plasma or serum levels of tumor necrosis factor-α (TNF-α) malondialdehyde (MDA), endotoxin (ET) and to discuss their significance in various types of viral hepatitis.
METHODS: PAF, TNF-α, MDA, and ET levels in 60 controls, 16 cases of acute viral hepatitis, 71 cases of chronic viral hepatitis, 19 cases of severe viral hepatitis were detected by reverse phase high-performance liquid chromatography (rHPLC), bio-assay, ELISA, thiobarbituric acid (TBA), and limulus lysate test (LLT), respectively.
RESULTS: The rHPLC was more sensitive and specific than bio-assay (r = 0.912, P<0.01). The plasma levels of PAF, TNF-α, MDA, and ET in patients with viral hepatitis were higher than those in controls (P<0.01).
CONCLUSION: rHPLC is more reliable and accurate for the detection of PAF.
- Citation: Cao HC, Chen XM, Xu W. Determination of platelet-activating factor by reverse phase high-performance liquid chromatography and its application in viral hepatitis. World J Gastroenterol 2005; 11(46): 7364-7367
- URL: https://www.wjgnet.com/1007-9327/full/v11/i46/7364.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i46.7364
Platelet-activating factor (PAF) is an autocoid mediator which controls intra- and extra-cellular signal transduction[1]. PAF is produced by a variety of cells including platelets, macrophages, neutrophils, and endothelial cells. Intrahepatic PAF is mainly secreted from Kupffer cells and hepatic sinusoidal endothelial cells. It activates platelets, leukocytes, and smooth muscle contraction. It was reported that PAF, as a mediator of lipid, plays an important role in the occurrence of hepatocyte injuries[2]. Direct infusion of PAF into the liver can result in the rapid release of superoxide and direct damage to liver cells[3,4]. PAF is also a mediator necessary for the development of endotoxin (ET) damage[5]. In endotoxic shock, the production of PAF in tissues is significantly increased. When the concentration of PAF is extremely low in the body, PAF manifests a short half-life of only 30 s and is then transformed rapidly into lyso-PAF without biological activity. PAF is hard to link to the carriers of protein due to its low immunogenicity, which makes the explanation difficult in the antibody preparation. Consequently, the current biological and immunological techniques cannot meet the clinical requirements[6]. Based on the biological determination, we used reverse phase high-performance liquid chromatography (rHPLC) to determine plasma PAF, tumor necrosis factor-α (TNF-α), malondialdehyde (MDA), and ET in patients with viral hepatitis and further clarified their effect and significance in all types of viral hepatitis in order to study the mechanism of viral hepatitis.
Between January 2001 and June 2002, 106 patients of both sexes diagnosed as viral hepatitis at the Department of Infectious Diseases of our hospital were recruited in the study. The patients consisted of 16 cases of acute hepatitis, 26 cases of chronic light hepatitis, 20 cases of chronic moderate hepatitis, 25 cases of chronic severe hepatitis (n = 25) and 19 cases of severe hepatitis, ranging in age from 40 to 61 years, with a mean age of 49.5±10.4 years. All the patients were diagnosed by the specialists of the Department of Infectious Diseases in accordance with the diagnostic criteria for viral hepatitis recommended on the 10th National Viral Hepatitis and Hepatopathy Conference in Xi’an 2000. All the patients did not receive any immunomodulators and any drugs within 2 wk prior to this study.
Healthy control subjects (n = 60) without any thrombotic or communicable diseases were recruited from the blood center.
Blood PAF levels were measured in patients and normal volunteers. We also measured the plasma or serum levels of TNF-α, ET, and MDA.
Agilent 1100 series HPLC, water chromatographic column (3.9 mm×150 mm), Lab-line® Aquawave™ ultrasonic cleaner, standard lyso-PAF-C16 and PAF-C18 (chromatographic grade) were from Sigma Company.
TNF-α was assayed with ELISA. The testing kits were from Jingmei Biological Engineering Co., Ltd.
ET was determined by limulus lysate test (LLT). The testing kits were from Shanghai Yi-Hua Clinical Medicine Technology Co., Ltd.
MDA was determined by thiobarbituric acid (TBA). The testing kits were from Nanjing Jian-Cheng Biological Engineering Research Institute.
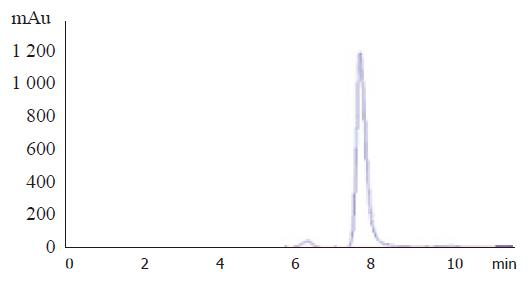
Plasma was separated from whole blood by centrifugation at 3 000 r/min for 10 min and then stored at -70 °C until assay. PAF was assayed as previously described[7]. The mobile phase was required according to chromatographic condition, the velocity of flow was 1.0 mL/min, the column temperature was at 25 °C (Figure 1) and the determination wave was 208 nm. The PAF values of 20 randomly selected samples were determined by the biological technique established in our laboratory.
The descriptive values of variables were expressed as mean±SD. Categorical data were compared between the two groups with the Cochran’s Q test. The correlations between laboratory results were evaluated by Pearson's correlation test. P<0.05 was considered statistically significant. All statistical analyses were performed by SPSS for Windows.
In the assay of PAF by rHPLC, the retention times of lyso-PAF and C18 PAF were 6.2 and 7.8 min, respectively. The correlation analysis was performed for the results in the chromatography group and the biological technique group (r = 0.912, P<0.01).
The ET value of over 50 ng/L represented the positive finding in endotoxemia detection[6]. The detection rate of endotoxemia was 51.9%, 55.9%, 67.8%, and 0% in the patients with acute hepatitis, chronic hepatitis, severe hepatitis, and in the controls, respectively. The positive detection rate in the patients was significantly higher than that in the controls (P<0.01).
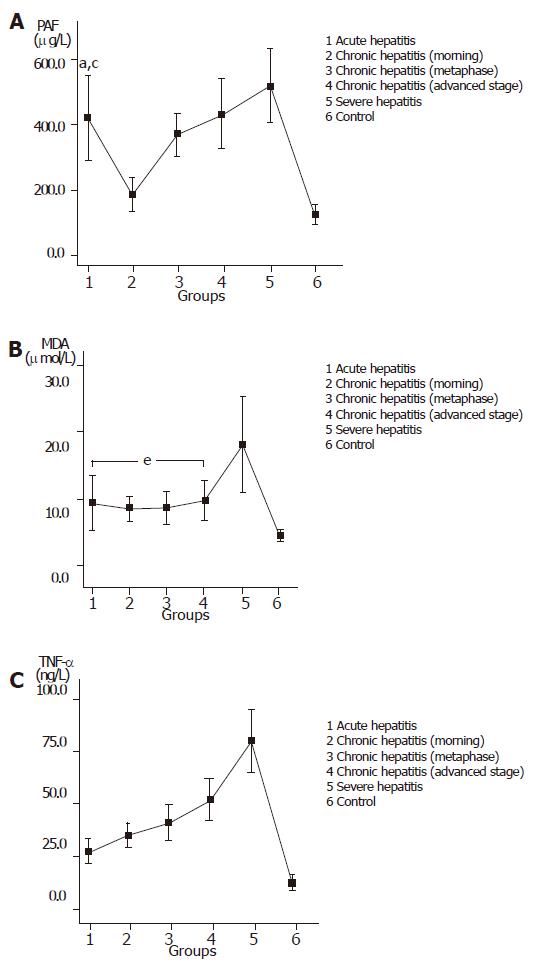
The values of PAF, MDA and TNF-α in the patients and controls are shown in Figures 2A-C.
The biological assay or radio immunoassay of PAF fails to manifest satisfactory specificity and sensitivity, because PAF in the body is rapidly transformed into lyso-PAF without biological activity[8]. In addition, the procedure is complicated and the results may be influenced by the individual difference of rabbit thrombocytes as well as the surgeon’s skill in plate preparation and sample application[9]. The immunoassay may also be limited by antibody preparation that is actually difficult to complete. A desirable correlation is indicated between rHPLC and biological assay. The assay of PAF by rHPLC can lead to the effective separation and then the assay of PAF components (including C16 and C18) with different structures in the assayed sample not related to the biological activity of PAF. Thus, the reference values obtained are notably higher than those obtained by biological assay, contributing to a great increase in accuracy.
The liver plays an important role in removing ET by Kupffer cells and macrophages[10]. In our study, the detection rate of endotoxemia was 52.80%, 56.41%, and 69.57% in acute, chronic and severe hepatitis, respectively. Besides direct damage to the liver, ET is able to induce and release a large amount of inflammatory mediators including PAF and TNF-α by activating Kupffer cells, macrophages and hepatic sinusoidal endothelial cells. These mediators are not only involved in various biological effects of ET, but also exhibit their own biological effects by inducing generation of other inflammatory mediators, resulting in inflammation and tissue injuries in the body by various mechanisms[11,12]. PAF may cause many symptoms of endotoxic shock, including hypotension, cardiac function inhibition, and blood plasma exudation. The specific receptor antagonist of PAF has preventive and therapeutic effects on endotoxic shock. ET also has certain synergistic effect on PAF. Animal experiments showed that simultaneous use of ET and PAF could lead to severe endotoxic shock, granulopenia, and bowel necrosis. The TNF-α gene expression induced by ET is also related to PAF. The PAF antagonist can be used to effectively reduce the level of TNF in blood plasma and partially inhibit intrahepatic synthesis of TNF mRNA, indicating that TNF mRNA may be synthesized in a PAF-dependent manner or in a PAF-independent manner[13].
MDA is generated through lipid peroxidation. It can damage the complete membrane structure of hepatocytes and organelles, activate Kupffer cells, lead to endotoxemia and is closely correlated with the occurrence of hepatopathy[14]. In this study, PAF in patients with acute hepatitis was notably higher than that in patients with early- or medium-phase chronic hepatitis, suggesting that ET, PAF, TNF, and MDA may result in the aggravation of liver injury[15]. The PAF antagonist perfusion into the liver in advance can increase bile production in the affected liver, decrease the production of lipid peroxides (including MDA) and prevent energy damage by PAF. The value of blood MDA is remarkably increased in the early stage of severe hepatitis and returns to the normal levels during convalescence (data not shown)[16]. Therefore, MDA can be used as one of the indicators of diagnosis and prognosis of severe hepatitis.
Co-first-authors: Hong-Cui Cao and Xiao-Ming Chen
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Cuschieri J, Bulger E, Garcia I, Jelacic S, Maier RV. Calcium/calmodulin-dependent kinase II is required for platelet-activating factor priming. Shock. 2005;23:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Grypioti AD, Theocharis SE, Papadimas GK, Demopoulos CA, Papadopoulou-Daifoti Z, Basayiannis AC, Mykoniatis MG. Platelet-activating factor (PAF) involvement in acetaminophen-induced liver toxicity and regeneration. Arch Toxicol. 2005;79:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Moy JA, Bates JN, Fisher RA. Effects of nitric oxide on platelet-activating factor- and alpha-adrenergic-stimulated vasoconstriction and glycogenolysis in the perfused rat liver. J Biol Chem. 1991;266:8092-8096. [PubMed] |
| 4. | Murohisa G, Kobayashi Y, Kawasaki T, Nakamura S, Nakamura H. Involvement of platelet-activating factor in hepatic apoptosis and necrosis in chronic ethanol-fed rats given endotoxin. Liver. 2002;22:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Watanabe J, Marathe GK, Neilsen PO, Weyrich AS, Harrison KA, Murphy RC, Zimmerman GA, McIntyre TM. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J Biol Chem. 2003;278:33161-33168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Ammit AJ, O'Neill C. Studies of the nature of the binding by albumin of platelet-activating factor released from cells. J Biol Chem. 1997;272:18772-18778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Cao HC, Xu WR, Zhu W, Chen XM, Li LJ. Determination of platelet-activating factor (PAF) by reversed phase high-performance liquid chromatographic technique (rHPLC) and its application in some elderly diseases. Linchuang Jianyan Zazhi. 2003;21:129-131. |
| 8. | Owen JS, Wykle RL, Samuel MP, Thomas MJ. An improved assay for platelet-activating factor using HPLC-tandem mass spectrometry. J Lipid Res. 2005;46:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Kita Y, Takahashi T, Uozumi N, Shimizu T. A multiplex quantitation method for eicosanoids and platelet-activating factor using column-switching reversed-phase liquid chromatography-tandem mass spectrometry. Anal Biochem. 2005;342:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Hiraoka A, Horiike N, Akbar SM, Michitaka K, Matsuyama T, Onji M. Expression of CD163 in the liver of patients with viral hepatitis. Pathol Res Pract. 2005;201:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Zhou F, Ajuebor MN, Beck PL, Le T, Hogaboam CM, Swain MG. CD154-CD40 interactions drive hepatocyte apoptosis in murine fulminant hepatitis. Hepatology. 2005;42:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Cao Q, Mak KM, Lieber CS. Cytochrome P4502E1 primes macrophages to increase TNF-alpha production in response to lipopolysaccharide. Am J Physiol Gastrointest Liver Physiol. 2005;289:G95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Souza DG, Teixeira MM. The balance between the production of tumor necrosis factor-alpha and interleukin-10 determines tissue injury and lethality during intestinal ischemia and reperfusion. Mem Inst Oswaldo Cruz. 2005;100 Suppl 1:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Liu LG, Yan H, Yao P, Zhang W, Zou LJ, Song FF, Li K, Sun XF. CYP2E1-dependent hepatotoxicity and oxidative damage after ethanol administration in human primary hepatocytes. World J Gastroenterol. 2005;11:4530-4535. [PubMed] |
| 15. | Buke AC, Buke M, Altuglu IE, Ciceklioglu M, Kamcioglu S, Karakartal G, Huseyinov A. Tumor necrosis factor alpha and interleukin 6 productions in response to platelet-activating factor in chronic hepatitis B virus infection. Med Princ Pract. 2004;13:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Eboumbou C, Steghens JP, Abdallahi OM, Mirghani A, Gallian P, van Kappel A, Qurashi A, Gharib B, De Reggi M. Circulating markers of oxidative stress and liver fibrosis in Sudanese subjects at risk of schistosomiasis and hepatitis. Acta Trop. 2005;94:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |