INTRODUCTION
Recently, a great deal of work has focused on a family of specific factors known as molecular chaperones. It has been established that molecular chaperones are involved in protein folding, assembly, disassembly, and degradation under normal conditions. It has also been established that molecular chaperones respond to stress states and certain disorders[1-4]. Previous studies demonstrated that Cpn60 was co-distributed with various secretory proteins in acinar cells of the mammalian exocrine pancreas and associated with pancreatic secretory proteins in the intracellular compartments involved in the secretion, such as the rough endoplasmic reticulum (RER), the Golgi (G), and the zymogen secretory granules (ZG). Cpn60 follows the RERGZG pathway in an increasing gradient as pancreatic enzymes[1,5,6]. The association of Cpn60 and pancreatic enzymes along the secretory pathway was confirmed by immunoprecipitation assay on isolated zymogen granule extracts, where amylase and lipase co-precipitated with Cpn60[5]. However, to our knowledge, there is little information available in literature about the relationship between Cpn60 and the enzymes change and also their significance in pancreatic pathogenesis. Therefore, streptozotocin-induced diabetes and deoxycholate-induced acute pancreatitis (AP) models in the Sprague-Dawley (SD) rats were replicated in order to explore the possible alterations of Cpn60 and pancreatic enzymes in the pancreatic acinar cells under the two experimental pathological conditions.
MATERIALS AND METHODS
Chemicals
Rabbit anti-Cpn60 was purchased from Stressgen, Victoria, BC, Canada. Rabbit anti-lipase, rabbit anti-chymotrypsinogen and protein A-gold complex were prepared in the laboratory as previously described[7,8]. Rabbit anti-amylase, sodium deoxycholate (NaDc), urethane and some other chemical reagents were purchased from Sigma Chemical Co., St. Louis, USA.
Animals and preparation of pathological models
SD male rats (n = 15, part from St. Charles River, Quebec, Canada and part from the Animal Center, Shanghai Medical College of Fudan University, China) were randomly divided into three groups: normal control group (n = 4), AP group (n = 6) and diabetes group (n = 5). (1) AP model: Six rats (each weighing 250 g) were fasted overnight with free access to water. They were anesthetized by an intraperitoneal injection of urethane at 1 g/kg body weight. AP was induced by a retrograde injection of 4% NaDc into the biliopancreatic duct (BPD) using a modified method of Zhang et al[9,10]. After a small median laparotomy, the BPD was temporarily closed at the liver hilum with a soft microvascular clamp to prevent reflux of the infused material into the liver. A retrograde injection of 4% NaDc into the distal BPD was performed (40 mg/kg body weight). The clamp was removed 5 min after the injection. The abdomen was closed and the rats were then kept for 5 h. (2) Diabetes model: Five rats were administered with a single intraperitoneal injection of streptozotocin dissolved in 10 mmol/L citrate buffer (pH 4.5), at 70 mg/kg body weight. The hyperglycemic state developed in the first 72 h after injection and remained throughout the 3 mo of the experiment.
Tissue processing
At the end of the experiments, all the rats were anesthetized with urethane and the splenic parts of the pancreases were removed and fixed by immersion with 10 g/L glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4) for 2 h at 4 °C. The tissue samples were washed with the phosphate buffer, dehydrated in a series of graded ethanol solutions, and embedded in Lowicryl K4M at -20 °C as described previously[7]. Ultrathin sections were cut, mounted on Parlodion-carbon-coated nickel grids, and processed for immunocytochemistry.
Immunocytochemistry
The ultrathin sections mounted on nickel grids were incubated by floating them on a drop of 1% ovalbumin in PBS (pH 7.2) for 10 min at room temperature (RT). They were then transferred separately to a drop of differently diluted primary antibodies for 2 h at RT (except 4 h for anti-amylase). The antibodies used here included rabbit anti-Cpn60 (1:10), rabbit anti-lipase (1:10), rabbit anti-chymotrypsinogen (1:10), and rabbit anti-amylase (1:500). The grids were rinsed with PBS, transferred to the ovalbumin solution for 10 min, and then incubated on a drop of the protein A-gold complex (10 nm in diameter)[6] for 30 min. They were then thoroughly washed with PBS and rinsed with distilled water. After staining with uranyl acetate, the sections were examined under a Philips 410 electron microscope. The specificity of each immunolabeling was tested effectively by two control experiments described previously[7].
Evaluation
Morphometrical evaluation of the labeling densities, as a reflection of the quantity, was performed using a Videoplan 2 image processing system. At least 30 micrographs, recorded at 28 000× final magnification, were analyzed for each animal tissue. Labeling densities were evaluated as described previously[6] and were reported as the mean of gold particles/μm2±SE to the mean. Statistical analyses of the results were carried out using the Student’s t-test. P<0.05 was considered statistically significant.
RESULTS
Distribution of Cpn60 and pancreatic enzymes in the pancreatic acinar cells of rats
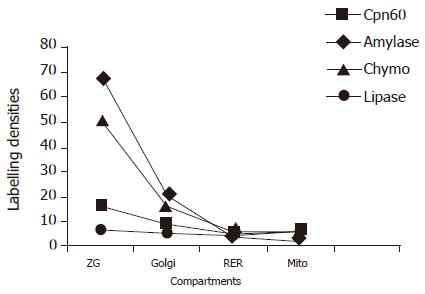
Immunocytochemistry labeling study on the sections of the pancreatic tissue revealed that in the acinar cells, both Cpn60 and the pancreatic enzymes, such as lipase, chymotrypsinogen, and amylase, co-existed in the RER, Golgi, and ZG. Increasing gradients of the labeling densities for both Cpn60 and the enzymes were present along the RER-G-ZG secretory pathway, with very low labeling in the mitochondria, in the normal rats and also in the experimental groups. (Figure 1).
Figure 1 Distribution of Cpn60 and pancreatic enzymes in the compartments of the pancreatic acinar cells in normal rats.
Labeling densities (gold particles/μm2) were measured by immunocytochemistry. ZG: zymogen granules; RER: rough endoplasmic reticulum; Mito: mitochondria; Chymo: chymotrypsinogen.
Changes of Cpn60 and pancreatic enzymes in the pancreatic acinar cells of diabetic rats
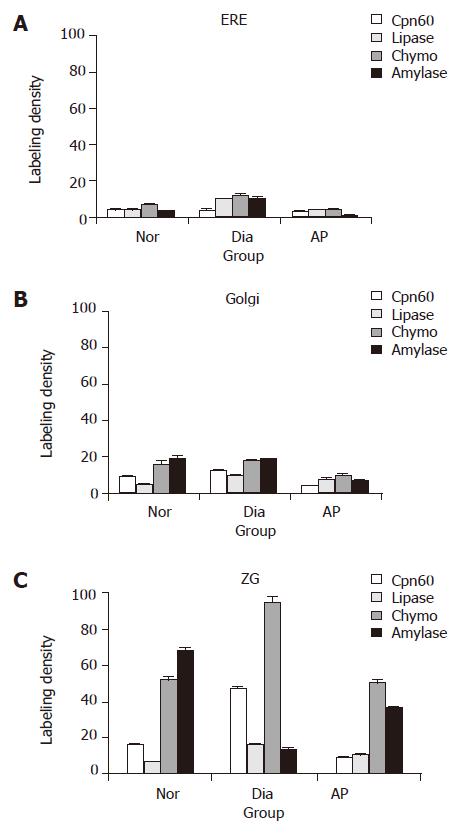
Compared with the normal group, the sections from the diabetic rats showed a significant increase in the Cpn60 labeling density, and also in the lipase and chymotrypsinogen labeling densities, especially in the zymogen granules of the pancreatic acinar cells (Figures 2 and 3). In addition, the immunolabelings of Cpn60 increased nearly 3-fold, lipase 2.5-fold, and chymotrypsinogen was twice as high as the normal group (P<0.05), but amylase level decreased drastically to a very low level (P<0.01 vs normal group, Figure 3).
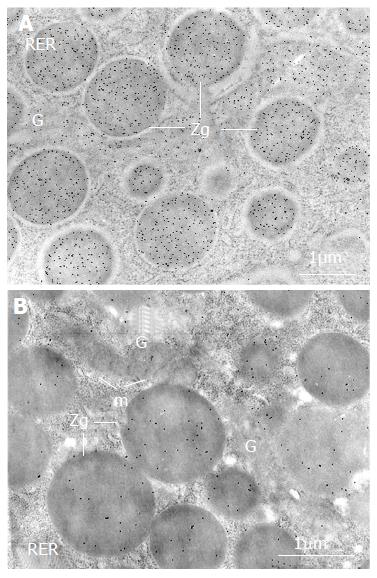
Figure 2 Changes of Cpn60 and pancreatic enzymes in the acinar cells of diabetic rats.
The labeling by gold particles (black particles), reflecting Cpn60 antigenic sites, is more intense over the zymogen granules (ZG) in the pancreatic acinar cell in diabetic rat (A) compared with that in normal rat (B). RER: rough endoplasmic reticulum; G: Golgi; m: mitochondria.
Figure 3 Immunolabeling densities of Cpn60 and the pancreatic enzymes in pancreatic acinar cells rats (particles/μm2, mean± SE).
A: Rough endoplasmic reticulum; B: Golgi; and C: zymogen granules. Nor: normal group; Dia: diabetic group; Chymo: chymotrypsinogen.
Changes of Cpn60 and pancreatic enzymes in the pancreatic acinar cells of AP rats
In AP, however, the labeling densities of Cpn60 declined through the secretory pathway (RER-G-ZG) of the pancreatic acinar cells (Figure 3), more significant in the zymogen granules where Cpn60 content decreased about 40% as compared with the normal group (P<0.05). However, lipase markedly increased and chymotrypsinogen was kept at a high level in the compartment, suggesting a nonparallel change with Cpn60 (Figure 3).
DISCUSSION
Molecular chaperones, including chaperonins (Cpn60 and Cpn10), heat shock proteins (Hsps) and other components are a large family of specialized proteins present in various cellular compartments. Some of them play important roles in enabling polypeptides to reach biologically active forms by serving as “detergents” and “proofreading apparatus” during protein synthesis and secretion[11]. In pancreatic acinar cells, like pancreatic enzymes, Cpn60 follows the well-characterized secretory pathway, sequentially increasing along the RER-G-ZG secretory pathway, and finally being discharged into the pancreatic juice[12,13]. In our former work, we have explored and analyzed the characteristic distribution of Cpn60 in the pancreatic acinar cells of rats, and an association of Cpn60 with lipase, amylase, and trypsinogen in the secretory pathway of normal rats[1,5,6].
The interesting finding, in this study, was that lipase and chymotrypsinogen increased to almost two to three times their normal levels in the acinar cells of the diabetic rats. However, the increased enzymes were not aggregated and autoactivated within the acinar cells like what happened in AP. The acinar cells and the pancreatic tissues were not damaged in this situation. The important protective mechanism may be associated with enough chaperon capacity where Cpn60 content increased significantly (nearly threefold) in the pathological condition. In this regard, it has been reported that Cpn60 is involved in successful folding, sorting, and assembly of pancreatic oligomeric polypeptides, and prevents aggregation of the premature polypeptides[14-16]. In addition, the increase of lipase and chymotrypsinogen and the decrease of amylase in diabetes may represent an adaptive process in which the diabetic body reduced starch digestion and then glucose absorption, and exhausted the fat and protein as the energy sources. In this regard, Nagy et al[17] found a temporarily increased lipase content, when plasma free fatty acid concentration was elevated. The present results demonstrated that the pancreatic lipase and chymotrypsinogen were affected by the body metabolism, and confirmed the complicated correlation between the hormones, enzymes, and the metabolic situation of glucose, fat, and proteins in the body.
AP is caused by different etiologic factors, and each of them affects firstly the pancreatic acinar cells, resulting in the intracellular activation of trypsinogen, and then the lipolytic enzymes which, in turn, injure the acinar cells and the whole pancreas[18,19]. The autodigestion of the pancreas by its own prematurely activated digestive proteases has been considered as an important event in the occurrence of AP[20,21], but the mechanism is under elucidation. Recently, Lee et al[22] found that water immersion stress induced Hsp60 expression, prevented intrapancreatic trypsinogen activation, and then protected against cerulein-induced rat pancreatitis. Other reports also demonstrated that stresses, such as heat, acid, and osmotic shock, induced an increased transcription and production of Cpn60, and showed a protection for the pancreas, which was considered as an “adaptive cytoprotection”[23-25]. It is well known that in the early stage of AP, progressive increases of amylase, trypsin, and lipase levels are found in the ascites and the plasma (hyperenzymemia), and there is also an increase of enzyme content in the individual acinar cells[26,27].
In the present study, increased labeling densities of lipase and chymotrypsinogen were displayed in the AP sections. As an adaptive cytoprotection, the level of Cpn60 should increase; however, the data showed a decreased Cpn60 level and implied an insufficient chaperone capacity. This could in fact alter the steady-state equilibrium between some pancreatic secretory enzymes and chaperones, thus initiating premature enzyme aggregation and autoactivation in acinar cells. In this regard, Strowski et al[28] found that the pancreas reacted to various kinds of stresses with different inductions of Hsp mRNAs during cerulein-induced AP, and they hypothesized that failure to appropriately increase Hsp levels in response to high doses of cerulein might be a factor involved in the development of pancreatitis. The results of our present study also support this hypothesis.
In summary, the changes in Cpn60 levels coincide with the changes of pancreatic enzyme levels, mostly with lipase, chymotrypsinogen in the pancreatic acinar cells. The equilibrium between Cpn60 and the pancreatic enzymes in the acinar cells breaks in AP, and Cpn60 content decreases, thereby suggesting an insufficient chaperone capacity. This may promote pancreatic premature enzyme autoactivation and play a role in the AP pathogenesis. The findings suggest that replenishing Cpn60 and increasing chaperone capacity may be a new therapeutic clue of AP.