Published online Dec 14, 2005. doi: 10.3748/wjg.v11.i46.7261
Revised: July 2, 2005
Accepted: July 8, 2005
Published online: December 14, 2005
AIM: To assess the usefulness of urinary trypsinogen-2 test strip, urinary trypsinogen activation peptide (TAP), and serum and urine concentrations of the activation peptide of carboxypeptidase B (CAPAP) in the diagnosis of acute pancreatitis.
METHODS: Patients with acute abdominal pain and hospitalized within 24 h after the onset of symptoms were prospectively studied. Urinary trypsinogen-2 was considered positive when a clear blue line was observed (detection limit 50 μg/L). Urinary TAP was measured using a quantitative solid-phase ELISA, and serum and urinary CAPAP by a radioimmunoassay method.
RESULTS: Acute abdominal pain was due to acute pancreatitis in 50 patients and turned out to be extrapancreatic in origin in 22 patients. Patients with acute pancreatitis showed significantly higher median levels of serum and urinary CAPAP levels, as well as amylase and lipase than extrapancreatic controls. Median TAP levels were similar in both groups. The urinary trypsinogen-2 test strip was positive in 68% of patients with acute pancreatitis and 13.6% in extrapancreatic controls (P<0.01). Urinary CAPAP was the most reliable test for the diagnosis of acute pancreatitis (sensitivity 66.7%, specificity 95.5%, positive and negative predictive values 96.6% and 56.7%, respectively), with a 14.6 positive likelihood ratio for a cut-off value of 2.32 nmol/L.
CONCLUSION: In patients with acute abdominal pain, hospitalized within 24 h of symptom onset, CAPAP in serum and urine was a reliable diagnostic marker of acute pancreatitis. Urinary trypsinogen-2 test strip showed a clinical value similar to amylase and lipase. Urinary TAP was not a useful screening test for the diagnosis of acute pancreatitis.
- Citation: Sáez J, Martínez J, Trigo C, Sánchez-Payá J, Compañy L, Laveda R, Griñó P, García C, Pérez-Mateo M. Clinical value of rapid urine trypsinogen-2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis. World J Gastroenterol 2005; 11(46): 7261-7265
- URL: https://www.wjgnet.com/1007-9327/full/v11/i46/7261.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i46.7261
Most patients with acute pancreatitis have mild and self-limited disease that resolves spontaneously, but about 20% of attacks are severe, with a mortality of about 10-25%[1]. Early diagnosis of acute pancreatitis is crucial to ensure rapid and appropriate treatment. However, the clinical features of acute pancreatitis can be difficult to distinguish from those of other acute abdominal conditions, and the diagnosis may be overlooked at first. Determination of amylase in serum or urine is the principal laboratory method for diagnosing acute pancreatitis. However, hyperamylasemia may be absent in 19% of cases[2], and increases in pancreatic enzyme levels can occur in patients with acute abdominal pain of extrapancreatic in origin[3-5].
The pathophysiology of acute pancreatitis involves the activation of the pancreatic proenzymes. The activation of trypsinogen followed by the activation of other pancreatic zymogens occurs early in the course of pancreatitis, in proportion to the extent of pancreatic injury[6]. Concentrations of the activation peptide of trypsinogen (TAP) in urine and of the activation peptide of procarboxypeptidase B (CAPAP) in urine and serum have shown to be promise predictors of severity in acute pancreatitis[7,8]. However, the clinical value of these markers for the early diagnosis of acute pancreatitis is unclear. Until now, a previous study of urinary TAP levels[7] and three other studies of serum and urine CAPAP[9-11] have shown significantly higher concentrations of these peptides in acute pancreatitis compared to non-pancreatic abdominal pain, therefore, suggesting the usefulness of these assays for diagnosing acute pancreatitis. On the other hand, qualitative rapid urine trypsinogen-2 test strip is easy to perform and has been shown to be a reliable and useful screening test for acute pancreatitis in daily practice[12-16], particularly in healthcare units lacking laboratory facilities.
This study was conducted to assess the usefulness of urinary trypsinogen-2 test strip, urinary TAP, and serum and urine CAPAP levels compared with conventional enzymes (serum amylase and lipase) for diagnosing acute pancreatitis.
All consecutive patients with acute abdominal pain admitted to the Section of Gastroenterology of an Acute-care University Hospital in Alicante, Spain, in an 8-month period, participated in an observational, prospective, cohort study. Admission to the hospital within 24 h of symptom onset was the criterion for inclusion. The diagnosis of acute pancreatitis was based on (1) typical clinical symptoms and at least a threefold increase of serum amylase, once other causes of abdominal pain had been excluded, and (2) evidence of pancreatic inflammation by imaging studies and/or surgery. The severity of acute pancreatitis was assessed according to the criteria established at the International Symposium on Acute Pancreatitis in Atlanta in 1992[17]. According to our protocol, a CT scan was performed in patients with severe acute pancreatitis, with unknown etiology and whenever it was necessary to establish the diagnosis of acute pancreatitis. In all the patients, blood and urine samples were collected within the first 24 h of hospitalization. The Hospital General Universitario Ethic’s Committee approved the protocol, and all patients gave their informed consent for the inclusion in the study. Patients were divided into two groups according to the origin of acute abdominal disease. There were 50 patients with acute pancreatitis (25 men, 25 women, mean age 62.5±16.5 years) and 22 patients (11 men, 11 women, mean age 50.8±22.4 years) whose acute abdominal pain turned out to be extrapancreatic in origin. A CT scan was performed in all the 22 patients with non-pancreatic abdominal pain to rule out the possibility of acute pancreatitis.
Serum amylase and lipase concentrations were measured by routine methods of our laboratory and for the remaining diagnostic markers, urine and serum samples were frozen immediately after collection and stored at (-20 °C) until analysis.
Serum amylase concentrations were measured using an enzymatic assay (Amyl, Boehringer Mannheim Systems, Mannheim, Germany). For this assay the limit of detection is 3 U/L and the range of the standard curve was comprised between 3 and 1 200 U/L. The reference range of serum amylase, previously established in our laboratory, was 26-100 U/L. Serum lipase was measured using a colorimetric enzymatic technique (Lip, Boehringer Mannheim Systems, Mannheim, Germany). For this assay the limit of detection was 3 U/L and the range of the standard curve was comprised between 3 and 300 U/L. The reference range of serum lipase, previously established in our laboratory, was 13-60 U/L.
Serum and urine CAPAP concentrations were measured using a radioimmunoassay method (CAPAP RIA kit, Euro-Diagnostica, Malmö, Sweden). The kit is based on a competitive radioimmunoassay using antibodies against human CAPAP. CAPAP both in standards and samples compete with 125I-labeled CAPAP in binding to the antibodies. The 125I-CAPAP binds to the antibodies in an inverse proportion to the concentrations of CAPAP both in standards and samples. Antibody-bound 125I-CAPAP is separated from the unbound fraction using the double antibody solid-phase technique. The radioactivity of the pellets was then measured. For this assay, the lower limit of detection was about 0.4 nmol/L and the range of the standard curve was comprised between 0 and 20 nmol/L. When the results above this limit were obtained, serum samples were diluted at 1:5 and urine samples at 1:10 were processed again.
Urinary TAP was measured using a quantitative solid-phase ELISA based on the competition between free and immobilized peptide binding to an antibody to TAP (TAPKIT, Biotrin International Ltd, Dublin, Ireland). Urine samples were collected in sterile containers with EDTA (at final concentration 5-10 nmol/L) and diluted to a ratio of 1:4 in the assay buffer according to the manufacturer’s instructions. TAP linked to a carrier protein is immobilized on the solid phase to which the peptide calibrator or diluted (1:4) urine sample, plus the TAP antibody, was added. After the reaction, the amount of antibody bound to the solid phase was measured spectrophotometrically (450 nm) after sequential addition of rabbit antibody to IgG-biotin conjugate, streptavidin-horseradish peroxidase, and tetramethyl-benzidine. The lower detection limit was 0.2 nmol/L (0.8 nmol/L in the 1:4 diluted samples) and the range of the standard curve was comprised between 0.14 and 200 nm.
The Actim Pancreatitis test strip (Medix Biochemica, Finland) for urinary trypsinogen-2 is an immunochromatographic test. After the test strip has been dipped into the urine sample, trypsinogen-2 is bound to monoclonal antibody-labeled blue latex particles, which migrate across a nitrocellulose membrane with a zone containing another antibody specific for another epitope on trypsinogen-2. At trypsinogen-2 concentrations higher than 50 μg/L, a blue line develops in this zone. The test was considered positive when a clear blue line was detected within 5 min. A control line was used to indicate proper functioning of the strip. If the control line was undetectable the assay was repeated.
Descriptive data were given as median with interquartile range (25th and 75th percentiles). The Mann-Whitney U test was used to compare the results between patients with acute pancreatitis and extrapancreatic controls. Statistical significance was set at P<0.05. Receiver operating characteristics (ROC) curves of serum and urine CAPAP levels and urinary TAP concentrations were used to determine the optimal cut-off levels. For serum concentrations of amylase and lipase, threefold increases in the reference values recommended by our laboratory were selected as cut-off values. Using these cut-off points, the sensitivity, specificity, positive and negative predictive values and positive likelihood ratio in establishing the diagnosis of acute pancreatitis were calculated. Comparison of different prognostic markers was made using the positive likelihood ratio for the different cut-off values that were selected. The SPSS/PC+10 statistical package on a personal computer was used for the analysis of data.
Of the 50 patients with acute pancreatitis, 15 (10 men and 5 women, mean age 66±16 years) had severe disease and 35 (17 men and 18 women, mean age 61±17 years) had mild disease. Etiology of pancreatitis and outcome are shown in Table 1. Local pancreatic complications included necrosis in 7 patients, pseudocyst in 4 and abscess in 1. Systemic complications consisted of renal failure in 6 patients, respiratory insufficiency in 6 and gastrointestinal hemorrhage in 5. Death occurred in 5 of 15 patients with severe pancreatitis. Diagnoses established in controls with non-pancreatic acute abdomen are shown in Table 2.
| Number of patients | Percentage | |
| Male/female | 25/25 | |
| Age, yr, mean (SD) | 62.5 (16.5) | |
| Etiology | ||
| Gallstones | 30 | 60 |
| Idiopathic | 12 | 24 |
| Alcohol | 3 | 6 |
| Post-ERCP | 3 | 6 |
| Hypertriglyceridemia | 2 | 4 |
| Severity | ||
| Severe | 15 | 30 |
| Mild | 35 | 70 |
| Complications | ||
| Local | 12 | 24 |
| Systemic | 17 | 34 |
| Death | 5 | 10 |
| Etiology | Number of patients | Percentage |
| Acute cholecystitis | 5 | 22.7 |
| Acute appendicitis | 3 | 13.6 |
| Coliky pain | 3 | 13.6 |
| Intestinal obstruction | 2 | 9.1 |
| Active Crohn’s disease | 2 | 9.1 |
| Acute cholangitis | 1 | 4.5 |
| Perforated peptic ulcer | 1 | 4.5 |
| Gastric carcinoma | 1 | 4.5 |
| Epiploic appendicitis | 1 | 4.5 |
| Abdominal abscess | 1 | 4.5 |
| Bleeding peptic ulcer | 1 | 4.5 |
| Mesenteric lymphadenitis | 1 | 4.5 |
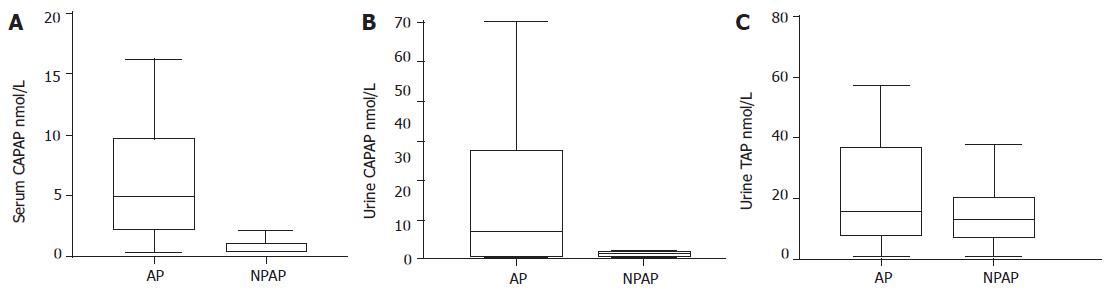
As compared with extrapancreatic controls, patients with acute pancreatitis had significantly higher values of amylase [942.20 (316.25-1 333.42) U/L vs 52 (29.50-91.20) U/L, P<0.001] and lipase [1 084 (228.75-2 133.75) U/L vs 22 (15.50-39) U/L, P<0.001] in serum. On the other hand, patients with acute pancreatitis showed significantly higher values of CAPAP in the serum and urine than controls with non-pancreatic acute abdomen, i.e., CAPAP in serum 5.05 (2.23-10.04) nmol/L vs 0.40 (0.40-1.14) nmol/L, P<0.001; CAPAP in urine 7.05 (0.93-29.5) nmol/L vs 1.36 (0.80-1.85) nmol/L, P<0.01) (Figures 1A and B). However, there were no statistically significant differences in urinary TAP between patients and controls [15.45 (7.79-37.05) nmol/L vs 12.70 (7.03-20.27) nmol/L] (Figure 1C). The urinary trypsinogen-2 test strip was positive in 68% of patients with acute pancreatitis and 13.6% in extrapancreatic controls (P<0.01).
Sensitivity, specificity, predictive values, pre-test probability, post-test probability and positive likelihood ratio for the different cut-off values of CAPAP in serum and urine, urinary TAP and trypsinogen-2 test are shown in Table 3. Urinary CAPAP was the most reliable test for the diagnosis of acute pancreatitis (sensitivity 66.7%, specificity 95.5%, positive predictive value 96.6%, negative predictive value 56.7% and post-test probability 97%), with a 14.6 positive likelihood ratio for a cut-off value of 2.32 nmol/L.
| Proenzyme, cut-off valuewithin 24 h of admission | Pre-testprobability% | Sensitivity% | Specificity% | PPV% | NPV% | Positivelikelihoodratio | Post-testprobability% |
| Amylase serum, >330 U/L | 69.4 | 74 | 86.4 | 92.5 | 59.3 | 5.4 | 92.4 |
| Lipase serum, >180 U/L | 69.4 | 84 | 85.7 | 93.4 | 72 | 5.87 | 93 |
| CAPAP serum, >1.53 nmol/L | 69.4 | 85 | 90.9 | 95.2 | 74 | 9.3 | 95 |
| CAPAP urine, >2.32 nmol/L | 69.4 | 66.7 | 95.5 | 96.9 | 56.7 | 14.6 | 97 |
| TAP urine, >10.01 nmol/L | 69.4 | 68.8 | 40 | 73.3 | 34.7 | 1.13 | 71.9 |
| Trypsinogen-2 urine, positive | 69.4 | 68 | 86.4 | 91.9 | 54.3 | 5 | 91.9 |
This study demonstrates that CAPAP in serum and urine (especially urinary CAPAP) was a reliable diagnostic marker of acute pancreatitis providing slightly better results than serum amylase and lipase. Urinary trypsinogen-2 test strip showed a clinical value similar to amylase and lipase. Urinary TAP was not a useful screening test for the diagnosis of acute pancreatitis. In agreement with previous studies[18,19], we also found that serum amylase and lipase were adequate diagnostic markers of acute pancreatitis.
In respect to urinary TAP concentrations, the present results are consistent with other studies[20,21] in which this marker was not useful for diagnosing acute pancreatitis. However, in the study of Neoptolemos et al[7], there were statistically significant differences in urinary TAP levels between patients with acute pancreatitis and controls both at 24-48 h of hospital admission and 24-48 h after the onset of symptoms. It may be possible that the increase in urinary TAP levels at 48 h compared to 24 h and the high rate of alcoholic pancreatitis included in the study of Neoptolemos et al[7] may account for the differences observed with the present findings.
With regard to clinical value of serum and urine CAPAP concentrations, both assays were reliable early markers for diagnosing acute abdominal pain of pancreatic origin. These results are similar to previous findings by our group in a series of 22 patients[22] and confirm that this marker of acute pancreatitis is capable of both simultaneously diagnosing and assessing severity of disease at the time of admission to the hospital[8-11]. Certainly, we do not know why urinary TAP was not a useful screening test for the diagnosis of acute pancreatitis whereas urinary CAPAP levels were a reliable test, since both assays indicate an activation of pancreatic zymogens. Probably, the different kinetics of release and techniques of determination of both markers could explain this point. More studies are necessary to assess it. On the other hand, laborious and time consuming work are pitfalls of current CAPAP assays for rapid screening in a routine diagnostic setting. Moreover, it hardly improves the diagnostic accuracy of the routine markers (amylase and lipase).
Rapid urine trypsinogen-2 strip test has been introduced in an effort to decrease the number of misdiagnosed cases of acute pancreatitis in an emergency setting. In the present study, the test was positive in 68% of patients with acute pancreatitis and 13.6% in extrapancreatic controls, with a sensitivity of 68% and specificity of 86.4%. These results are slightly less favorable than those reported in other series[12-16], in which 90% sensitivity was almost reached. However, it should be noted that a high percentage of patients with non-pancreatic acute abdominal pain with a clinical profile similar to acute pancreatitis (cholecystitis five cases, biliary colic three cases) were included in our study. Therefore, the present results far from decreasing the clinical usefulness of urinary trypsinogen-2 test strip, contribute to delimit the true value of this rapid screening test in clinical practice. On the other hand, it has to be considered that the inclusion process of patients with abdominal pain in this investigation may be biased, since patients admitted to our department have been pre-selected by the emergency department, and this likely justifies the high proportion of patients with AP in our series (50/72).
In summary, in patients with acute abdominal pain, hospitalized within 24 h of symptom onset, CAPAP in serum and urine was a reliable diagnostic marker of acute pancreatitis, but it provided only slightly better results than serum amylase and lipase and, although we have not made a detailed economical analysis, it seems not to be cost-effective to use it as a routine diagnostic marker. On the other hand, urinary trypsinogen-2 test strip showed a clinical value similar to amylase and lipase. Urinary TAP was not a useful screening test for the diagnosis of acute pancreatitis.
We thank Marta Pulido, MD, for editing the manuscript and editorial assistance.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Dervenis C, Johnson CD, Bassi C, Bradley E, Imrie CW, McMahon MJ, Modlin I. Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini consensus conference. Int J Pancreatol. 1999;25:195-210. [PubMed] |
| 2. | Clavien PA, Robert J, Meyer P, Borst F, Hauser H, Herrmann F, Dunand V, Rohner A. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Ann Surg. 1989;210:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Dutta SK, Douglass W, Smalls UA, Nipper HC, Levitt MD. Prevalence and nature of hyperamylasemia in acute alcoholism. Dig Dis Sci. 1981;26:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Yadav D, Nair S, Norkus EP, Pitchumoni CS. Nonspecific hyperamylasemia and hyperlipasemia in diabetic ketoacidosis: incidence and correlation with biochemical abnormalities. Am J Gastroenterol. 2000;95:3123-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Terada T, Nakanuma Y. Immunohistochemical demonstration of pancreatic alpha-amylase and trypsin in intrahepatic bile ducts and peribiliary glands. Hepatology. 1991;14:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Borgström A, Appelros S. Activation peptides in acute pancreatitis. In: Büchler MW, Uhl W, Friess H, Malfertheiner P, ed. Acute pancreatitis. Novel concepts in biology and therapy. Blackwell Wissenschafts-Verlag, Berlin-Viena 1999; 219-223. |
| 7. | Neoptolemos JP, Kemppainen EA, Mayer JM, Fitzpatrick JM, Raraty MG, Slavin J, Beger HG, Hietaranta AJ, Puolakkainen PA. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet. 2000;355:1955-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 337] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Appelros S, Petersson U, Toh S, Johnson C, Borgström A. Activation peptide of carboxypeptidase B and anionic trypsinogen as early predictors of the severity of acute pancreatitis. Br J Surg. 2001;88:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Pezzilli R, Morselli-Labate AM, Barbieri AR, Platè L. Clinical usefulness of the serum carboxypeptidase B activation peptide in acute pancreatitis. JOP. 2000;1:58-68. [PubMed] |
| 10. | Ung CT, Westlake S, Brennan H, Johnson CD. Activation peptide of carboxy peptidase B (CAPAP) for prediction of complicated acute pancreatitis (abstract). Digestion. 2000;61:289. |
| 11. | Müller CA, Appelros S, Uhl W, Büchler MW, Borgström A. Serum levels of procarboxypeptidase B and its activation peptide in patients with acute pancreatitis and non-pancreatic diseases. Gut. 2002;51:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Hedström J, Korvuo A, Kenkimäki P, Tikanoja S, Haapiainen R, Kivilaakso E, Stenman UH. Urinary trypsinogen-2 test strip for acute pancreatitis. Lancet. 1996;347:729-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kemppainen EA, Hedström JI, Puolakkainen PA, Sainio VS, Haapiainen RK, Perhoniemi V, Osman S, Kivilaakso EO, Stenman UH. Rapid measurement of urinary trypsinogen-2 as a screening test for acute pancreatitis. N Engl J Med. 1997;336:1788-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Kylänpää-Bäck M, Kemppainen E, Puolakkainen P, Hedström J, Haapiainen R, Perhoniemi V, Kivilaakso E, Korvuo A, Stenman U. Reliable screening for acute pancreatitis with rapid urine trypsinogen-2 test strip. Br J Surg. 2000;87:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Pezzilli R, Morselli-Labate AM, d'Alessandro A, Barakat B. Time-course and clinical value of the urine trypsinogen-2 dipstick test in acute pancreatitis. Eur J Gastroenterol Hepatol. 2001;13:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kemppainen E, Hedström J, Puolakkainen P, Halttunen J, Sainio V, Haapiainen R, Stenman UH. Urinary trypsinogen-2 test strip in detecting ERCP-induced pancreatitis. Endoscopy. 1997;29:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1734] [Article Influence: 54.2] [Reference Citation Analysis (1)] |
| 18. | Gumaste VV, Roditis N, Mehta D, Dave PB. Serum lipase levels in nonpancreatic abdominal pain versus acute pancreatitis. Am J Gastroenterol. 1993;88:2051-2055. [PubMed] |
| 19. | Steinberg WM, Goldstein SS, Davis ND, Shamma'a J, Anderson K. Diagnostic assays in acute pancreatitis. A study of sensitivity and specificity. Ann Intern Med. 1985;102:576-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 105] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Fernández-del Castillo C, Harringer W, Warshaw AL, Vlahakes GJ, Koski G, Zaslavsky AM, Rattner DW. Risk factors for pancreatic cellular injury after cardiopulmonary bypass. N Engl J Med. 1991;325:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 167] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Banks PA, Carr-Locke DL, Slivka A, Van Dam J, Lichtenstein DR, Hughes M. Urinary trypsinogen activation peptides (TAP) are not increased in mild ERCP-induced pancreatitis. Pancreas. 1996;12:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |