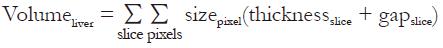Published online Dec 14, 2005. doi: 10.3748/wjg.v11.i46.7248
Revised: June 23, 2005
Accepted: July 1, 2005
Published online: December 14, 2005
AIM: To explore the role of the adaptor molecule in liver regeneration after partial hepatectomy (PH).
METHODS: We used transgenic mice expressing an N-terminal truncated form of MORT1/FADD under the control of the albumin promoter. As previously shown, this transgenic protein abrogated CD95- and CD120a-mediated apoptosis in the liver. Cyclin A expression was detected using Western blotting. ELISA and RT-PCR were used to detect IL-6 and IL-6 mRNA, respectively. DNA synthesis in liver tissue was measured by BrdU staining.
RESULTS: Resection of 70% of the liver was followed by a reduced early regenerative response in the transgenic group at 36 h. Accordingly, 36 h after hepatectomy, cyclin A expression was only detectable in wild-type animals. Consequently, the onset of liver mass restoration was retarded as measured by MRI volumetry and mortality was significantly higher in the transgenic group.
CONCLUSION: Our data demonstrate for the first time an involvement of the death receptor molecule MORT1/FADD in liver regeneration, beyond its well described role as part of the intracellular death signaling pathway.
- Citation: Schuchmann M, Rückert F, Garcia-Lazaro JF, Karg A, Burg J, Knorr N, Siebler J, Varfolomeev EE, Wallach D, Schreiber W, Lohse AW, Galle PR. MORT1/FADD is involved in liver regeneration. World J Gastroenterol 2005; 11(46): 7248-7253
- URL: https://www.wjgnet.com/1007-9327/full/v11/i46/7248.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i46.7248
Liver regeneration upon extensive liver damage is critical to survive diseases, such as fulminant hepatitis without liver transplantation. Beyond the cause of the underlying liver disease, it is well accepted that death receptor-mediated hepatocyte apoptosis is an important mechanism for liver damage[1,2]. The cascade of intracellular signaling events during apoptosis is reasonably well understood. Of critical importance is the activation of caspases, the subsequent cleavage of different death substrates and finally the disintegration of the cell. In this setting, MORT1/FADD is a central adaptor molecule for a number of death receptors to recruit and activate the initiator caspase-8 upon ligand-mediated aggregation[3,4]. Gene targeting of MORT1/FADD[5,6] or expression of a dominant negative mutant[7,8] confers resistance to CD95-mediated apoptosis in T cells. Recently, we have demonstrated that MORT1/FADD is also pivotal for CD95- and TNF-mediated apoptosis in hepatocytes in vivo[9]. In addition, emerging evidence suggests that death receptors and their intracellular signaling proteins have a dual role and may also be involved in the regeneration and repair mechanisms. TNF receptor I (CD120a) has been found to induce proliferation in a number of cells[10] and is involved in the initiation of liver regeneration[11]. Even the prototypical death receptor CD95 was described to exert non-apoptotic functions[12], e.g., stimulation of liver regeneration after partial hepatectomy (PH)[13]. In line with this observation, mice with the lpr genotype and decreased expression of CD95 showed a delayed regenerative response after PH[13]. In addition, the most upstream signaling molecules of the intracellular death pathway MORT1/FADD and caspase-8 have been described to be critical for T-cell proliferation[5,6,14]. To our knowledge, the mechanism by which MORT1/FADD contributes to T-cell proliferation is not completely elucidated yet. The regulatory function of MORT1/FADD seems to be structurally independent of the death domain and its role in apoptosis, but to rely on phosphorylation at serine residue S191, which has been shown to be critical for the regulative role of MORT1/FADD in cell proliferation[15].
The role of MORT1/FADD in liver regeneration has not been described so far. We used transgenic mice expressing a dominant negative mutant of MORT1/FADD in a liver-specific manner to investigate a possible role of the adaptor molecule in liver regeneration. We observed a delayed onset of liver proliferation upon PH compared to wild-type littermates and found a higher early postoperative mortality in dnMORT1/FADD transgenic mice. The increased rate of mortality might be due to initially retarded liver regeneration and underlines the critical role of MORT1/FADD in the complex network of signaling proteins which orchestrate liver regeneration.
Heterozygous transgenic mice (strain CB6F1) expressing liver-specific dominant negative mutant of the adaptor protein MORT1/FADD under the control of the albumin promoter were generated as previously described[9]. All animals were bred at the animal facility of the University of Mainz, had ad libitum access to water and food under standard conditions with 12-h dark/light cycle. All experiments were done in accordance with the Federal law and were approved by the Local Committee for Experimental Animal Research.
Male transgenic and wild-type animals at 6-8 wk of age were fasted overnight and operated in the morning. Mice were anaesthetized with subcutaneous injection of a solution containing 0.16% xylazinum and 1.2 mg ketamin (200 µL/20 g body weight). A 70% PH was performed by ligating and removing the left and median lobe at its root. The mice were killed at different time points and livers were harvested. The livers were either shock-frozen in liquid nitrogen and stored at -80 °C or fixed at 4 °C in 40 g/L paraformaldehyde overnight for further use. Serum was obtained by heart puncture.
After PH, the mice were treated at different time points with 1 mmol/L BrdU (10 g/kg body weight) 3 h prior to killing. The livers were harvested and shock-frozen. The livers were cut into 5-µm-thick slides with a microtome. BrdU incorporation was measured using the In situ Cell Proliferation Kit (Roche Diagnostics GmbH, 82372 Penzberg, Germany) according to the manufacturer's instructions. Results were presented as an average percentage of BrdU-positive cells counted in at least two fields of each slide under 10× high power field. Mitosis of the was counted by two independent observers in a blinded fashion.
Cytokine concentrations were assessed using ELISA, OptEIA Mouse IL-6 Set (Pharmingen, BD Biosciences, 69126 Heidelberg, Germany) following manufacturer's instructions. Samples were analyzed with an Elisa reader (MRX TC II, Dynex Technologies Lim., West Sussex BN).
Western blot analysis was performed with whole liver extract lysed in a lysis buffer. Protein concentration was equilibrated using Bradford assay reagent. The samples were boiled with sodium dodecyl sulfate sample buffer and electrophoresed on a 100 g/L sodium dodecyl sulfate-polyacrylamide gel. Following electrophoresis, the samples were blotted onto a PVDF-membrane (Pall, Germany), and the membrane was blocked with 20 g/L milk powder for 30 min, followed by an overnight incubation with a rabbit anti-cyclin A serum (Anti-rabbit polyclonal Cyc A C-19, # sc596, Santa Cruz) at 4 °C with phosphate-buffered saline/Tween (0.1%) with 20 g/L milk powder (PBSTM). After washing, the membranes were incubated for 45 min with horseradish peroxidase-conjugated goat-anti-rabbit serum (dilution 1:3 000) in PBSTM. Blots were developed using the Western Lightning Chemiluminescence Reagent (PerkinElmer Life Sciences, Boston, MA, USA).
Magnetic resonance imaging (MRI) was performed on a Magnetom Vision whole body scanner (Siemens Medical Solutions, Germany) at 1.5 T. The scanner was equipped with an experimental gradient system with maximum gradient field strength of 50 mT/m and a slew-rate of 160 mT/m/ms. A circular polarized small loop coil with 4 cm as diameter was used for imaging the mice.
For the imaging of the liver, a T1-weighed spinecho sequence with fat saturation and TR/TE/α = 640 ms/14 ms/90 °C was used. The experimental gradient system allowed a high resolution of 0.39 mm×0.39 mm with a slice thickness of 1 mm at a 50 mm×100 mm field of view. The gap between the slices was 0.8 mm.
Post processing was done using the Java-based free software ImageJ (National Institutes of Health, USA, download at http://rsb.info.nih.gov/ij). After drawing a region of interest (ROI) following the borders of the liver in every slice, the liver volume was calculated from the sum of pixels in each ROI over all slices with respect to the pixel size, the slice thickness and the slice gap is as follows:
Math 1
RNA was isolated from the cells using the First Strand cDNA Synthesis Kit for RT-PCR (Roche Diagnostics GmbH, 68305 Mannheim) according to the manufacturer's instructions. Primers for IL-6 were purchased from Metabion GmbH, D-82152 Planegg-Martinsried, Germany.
Duplicate PCR amplifications were carried out in a Light Cycler Fast Start DNA Master Green ITM (Roche Diagnostics GmbH, 68305 Mannheim) using Fast Start Light CyclerTM DNA Master containing Taq-polymerase, reaction buffer, and dNTPs (Roche). All reactions were performed in 10 µL volumes and fluorescence quantification was calculated with the aid of built-in Light Cycler software, version 3.01 (Roche).
For quantification of the mRNA, we chose the method of relative quantification with external standards (HPRT-specific primers).
Liver enzymes in serum were measured with a Roche Hitachi 917.
Liver samples were fixed overnight at 4 °C with 40 g/L paraformaldehyde. The tissue was then dehydrated, embedded in paraffin, and 5-µm sections were stained with HE. Mitosis of the cells was counted by two independent observers in blinded fashion under 10×high power fields (about 50 cells/field).
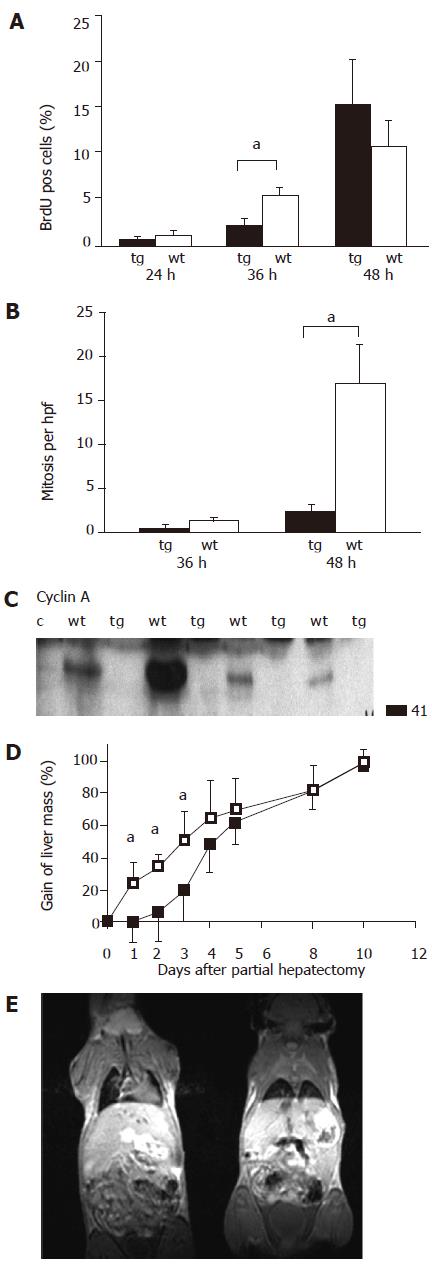
As previously described, PH was performed by resection of around 70% of the total liver volume. DNA synthesis as a marker of the regenerative response was measured by BrdU staining. Kinetics of BrdU staining after PH obviously differed between transgenic and wild-type animals (Figure 1A). We observed a significantly more BrdU-positive cells in wild-type animals after 36 h, which was attenuated after 48 h (Figure 1A). In line with this observation, the number of mitoses differed in regenerating liver 48 h after PH (Figure 1B). Differences were also observed in expression levels of cyclin A at 36 h after hepatectomy (Figure 1C), when the expression was detected only in wild-type mice. These observations were further substantiated by MRI technique (Figure 1E), which allowed to follow closely the kinetic of liver mass restoration after hepatectomy. Here we could demonstrate that the delayed onset of liver regeneration was paralleled by a retarded gain of liver volume (Figure 1D). After all, surviving transgenic animals finally also reached complete volume restoration around at d 10 after PH, as did wild-type animals.
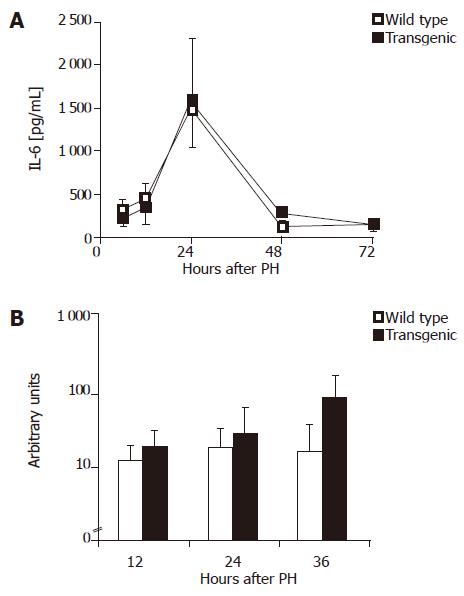
IL-6 has been shown to be an essential factor during initial liver regeneration[16]. Wuestefeld et al.[17] specified the effect of IL-6 and demonstrated an important hepatoprotective role of IL-6 during the early phase of liver regeneration. We therefore investigated possible differences in IL-6 levels after PH. Serum IL-6 levels peaked around 24 h after PH (Figure 2A), however, did not significantly differ between wild-type and transgenic animals. Interestingly, a significantly higher IL-6 mRNA expression was detected in transgenic livers 36 h after hepatectomy (Figure 2B). This observation demonstrated a functional IL-6 deficiency to be unlikely and rather pointed to a compensatory reaction towards delayed regeneration.
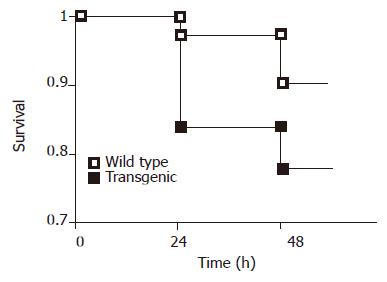
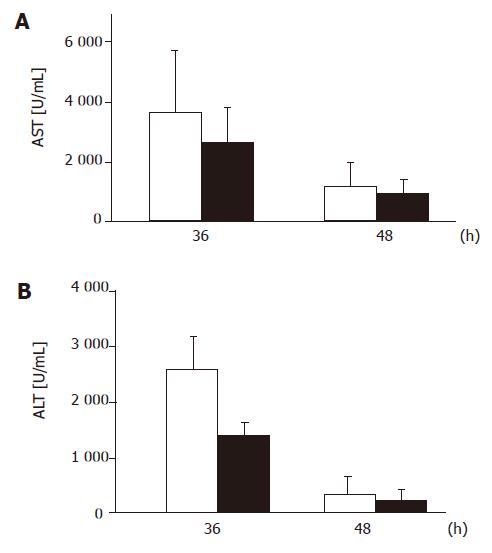
Surprisingly, we observed significantly higher early postoperative mortality after resection of 70% of the liver mass among the transgenic mice expressing the dominant negative MORT1/FADD mutant (Figure 3) compared to the wild-type littermates. We found a significant difference in postoperative mortality during the first 24 h, when 16.49% (13/81) of transgenic animals whereas, only 2.77% (2/72) of wild-type mice died (P<0.01, Fisher’s exact test). However, the mortality did not significantly differ later on. In order to elucidate the cause of different mortality rate, we measured liver enzymes during the early phase of regeneration. However, we did neither detect significant differences in liver function tests (Figure 4) nor differences in the degree of steatosis in liver sections at 36 or 48 h after PH (data not shown).
In the present study, we could show for the first time that MORT1/FADD acts as a multi-functional adaptor protein in the liver in vivo. In addition to its critical role in CD95- and CD120a-mediated liver failure[9], MORT1/FADD is involved in initiating liver regeneration upon PH. These results underline the dual role of MORT1/FADD, i.e., it was originally identified as an adaptor protein which is pivotal to convey death signals of the death receptors CD95 and CD120a[3,4] and the TRAIL receptors DR4 and DR5[18]. Initial evidence that the axis MORT1/FADD-caspase-8 has an additional role beside mediating apoptotic cell death revealed in the experiments with MORT1/FADD and caspase-8 knock out animals, showing that both genetic defects lead to embryonic lethality around d 11 in utero with similar pathology of cardiac malformation[5,6,19]. Interestingly, mice lacking functional flip/cash gene, a caspase-8 homolog without enzymatic activity, also died[20] in utero with a similarly impaired cardiac development. Later on, experiments with T-cells lacking MORT1/FADD or expressing a dominant negative mutant indicated an additional role of MORT1/FADD in the regulation of cell proliferation[5-8,21].
The exact mechanism by which MORT1/FADD contributes to cell proliferation is still enigmatic, especially when the involved downstream molecules are not identified. Phosphorylation of MORT1/FADD at position serine 191 in mouse (corresponding to serine 194 in human beings) seems to be of crucial importance[15,22]. Current data have substantiated the idea of a bifurcation of the signal pathway at the level of MORT1/FADD by either sending a death signal via activation of caspase-8 or signaling towards cell proliferation. FLIP, which is also recruited to the receptor complex, has been reported to modulate caspase-8-mediated cell death and is a good candidate for further delivering a proliferation signal[23]. Indeed, FLIP has been shown to be involved in T-cell proliferation[23]. However, it has not been completely understood how FLIP further mediates the proliferation signal leading to the activation of ERK[23]. The molecular architecture of the intracellular bifurcation is complex, since the lack of caspase-8 does not only provide deficiency in death receptor apoptosis signaling but also confers an impaired T-cells proliferation[14]. According to data of Iimuro et al.[24] and Chaisson et al.[25], NF-кB is not involved in the orchestration of the initial DNA synthesis after PH.
A regenerating liver is protected against CD95-induced cell death[26]. This might partially be attributed to the downregulation of CD95 but resetting the function of proapoptotic molecules, such as MORT1/FADD during the initial proliferative response might also contribute. It is not obvious which receptor engages MORT1/FADD to contribute to liver regeneration. CD120a (TNF-receptor 1) and CD95 are both candidates since TNF-R1 knock-out mice[11] as well as mice with the decreased hepatic CD95 expression (lpr-genotype)[13] showed a pronounced delay of liver regeneration after PH. In our study, the cause of a high postoperative mortality in transgenic mice remains unclear. We could not find signs of liver dysfunction in the early postoperative phase and there was also presence of IL-6 expression. We even detected higher intrahepatic IL-6 mRNA levels in the transgenic mice. A previous study also reported no difference between wild-type and transgenic animals upon LPS or CpG-DNA challenge[27].
In conclusion, we provide further evidence for a complex role of the adaptor molecule MORT1/FADD which is also involved in death and proliferation pathways in hepatocytes. This observation should be taken into consideration while targeting anti-apoptotic as well as anti-proliferative therapies.
The authors thank Sonja Bamberger, Sonja Klein and Nicole Voltz for expert technical assistance, Dr. Kurt Reifenberg for support in the animal facility and Regine Weisbrod M.A. for critically reading the manuscript. The data presented are part of the MD thesis of F.R.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 539] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 2. | Streetz K, Leifeld L, Grundmann D, Ramakers J, Eckert K, Spengler U, Brenner D, Manns M, Trautwein C. Tumor necrosis factor alpha in the pathogenesis of human and murine fulminant hepatic failure. Gastroenterology. 2000;119:446-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795-7798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 763] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 4. | Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1780] [Cited by in RCA: 1800] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 5. | Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 586] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 6. | Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954-1958 DOI : 10.1126/science.279.5358.1954. |
| 7. | Newton K, Harris AW, Bath ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 351] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Walsh CM, Wen BG, Chinnaiyan AM, O'Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Schuchmann M, Varfolomeev EE, Hermann F, Rueckert F, Strand D, Koehler H, Strand S, Lohse AW, Wallach D, Galle PR. Dominant negative MORT1/FADD rescues mice from CD95 and TNF-induced liver failure. Hepatology. 2003;37:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1391] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 11. | Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 753] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 12. | Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 635] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 13. | Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med. 2000;6:920-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 384] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Hua ZC, Sohn SJ, Kang C, Cado D, Winoto A. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity. 2003;18:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1211] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 17. | Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Müller W, Trautwein C. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003;278:11281-11288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Kuang AA, Diehl GE, Zhang J, Winoto A. FADD is required for DR4- and DR5-mediated apoptosis: lack of trail-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J Biol Chem. 2000;275:25065-25068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 932] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 20. | Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 394] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 21. | Zörnig M, Hueber AO, Evan G. p53-dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Curr Biol. 1998;8:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Alappat EC, Volkland J, Peter ME. Cell cycle effects by C-FADD depend on its C-terminal phosphorylation site. J Biol Chem. 2003;278:41585-41588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000;10:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 449] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 24. | Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 357] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002;110:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Takehara T, Hayashi N, Mita E, Kanto T, Tatsumi T, Sasaki Y, Kasahara A, Hori M. Delayed Fas-mediated hepatocyte apoptosis during liver regeneration in mice: hepatoprotective role of TNF alpha. Hepatology. 1998;27:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Schuchmann M, Hermann F, Herkel J, van der Zee R, Galle PR, Lohse AW. HSP60 and CpG-DNA-oligonucleotides differentially regulate LPS-tolerance of hepatic Kupffer cells. Immunol Lett. 2004;93:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |