Published online Dec 7, 2005. doi: 10.3748/wjg.v11.i45.7188
Revised: May 23, 2005
Accepted: May 24, 2005
Published online: December 7, 2005
AIM: To evaluate whether the cytokine responses in liver and serum differ in chronic hepatitis C patients with normal and high alanine aminotransferase (ALT) levels.
METHODS: Thirty-three (16 with normal ALT level as group 1 and 17 with elevated ALT level as group 2) patients infected with genotype 1b hepatitis C virus (HCV) were examined. Liver infiltrating lymphomononuclear cells (LILMCs) were isolated from liver biopsy by collagenase type 1 and stimulated with phytohemagglutinin and interleukin 2 (IL-2). IL-10, IL-12, interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) were determined in serum and LILMCs by ELISA.
RESULTS: Serum cytokine levels were similar in both groups (P>0.05). Stimulated IFN-γ and TNF-α levels in LILMCs were increased in both groups. IL-12 and IL-10 levels stimulated with IL-2 were higher in group 1 than in group 2 (P = 0.023). Histological activity index (HAI) and stage had a negative correlation with TNF-α and IFN-γ levels in group 2.
CONCLUSION: Increased T-helper type 2 (Th2) cytokine response may regress inflammatory and biochemical activity. Progression of histological abnormalities in persons with elevated ALT probably depends on insufficient Th2 cytokine response, which does not balance Th1 cytokine response.
- Citation: Akyüz F, Polat N, Kaymakoglu S, Aksoy N, Demir K, Beşışık F, Badur S, Çakaloglu Y, Ökten A. Intrahepatic and peripheral T-cell responses in genotype 1b hepatitis C virus-infected patients with persistently normal and elevated aminotransferase levels. World J Gastroenterol 2005; 11(45): 7188-7191
- URL: https://www.wjgnet.com/1007-9327/full/v11/i45/7188.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i45.7188
The liver is the primary site of hepatitis C virus (HCV) replication[1]. Clearance and control of HCV infection depend on immune responses. T lymphocytes and immunoregulatory cytokines play an important role in the host response to HCV infection[2]. Intrahepatic T-cell response to HCV may determine the hepatic injury of HCV infection[3,4]. T cells also play a role in HCV clearance[5]. Th1 cytokines such as IL-2 and IFN-γ are required for host antiviral immune response, while Th2 cytokine (IL-10) can inhibit the development of these responses[2]. TNF-α also triggers a partially overlapping set of antiviral defense mechanisms and serum level of TNF-α reflects the progression of inflammation[6,7].
The natural course of HCV infection is not clear. Also, the behavior of HCV infection cannot be estimated. Different immune responses in each patient may determine the clinical course. Approximately 30% of patients with chronic HCV infection show persistently normal ALT levels that are positive for HCV-RNA. Only a small number of these patients (0-20%) have normal histology. These people are referred as healthy HCV carriers[8]. Immunological differences between patients with normal and elevated ALT levels are not well known. Most patients with normal ALT levels have a certain degree of liver damage and show a slow progression[9].
This study aimed to evaluate whether the cytokine responses in liver and serum differ in chronic hepatitis C patients with normal and high ALT levels.
Thirty-three patients infected with genotype 1b HCV were studied. Sixteen of them had persistently normal ALT levels (group 1), while 17 had high ALT levels (group 2) by at least four analyses in a year. Anti-HCV was determined by UBI EIA 4.0 (Organon Teknika, Holland). HCV-RNA was determined by polymerase chain reaction (COBAS Amplicor 2, Roche Diagnostics, Germany) and HCV genotyping was carried out with INNO-LiPA HCV assay (Innogenetics NV, Belgium). Hepatitis B surface antigen (HBsAg) and anti-HIV were negative in all patients. HBsAg was detected by immunoenzymatic assay [Hepanostika HBsAg Uni-Form II (Organon Teknika, Holland)]. All patients were also negative for any other etiology of liver diseases. Blood was drawn from all patients and centrifuged at 2 500 r/min for 5 min and separated serum samples were stored at -85 °C. Liver biopsy was performed in all the patients before the treatment. Histological changes (HAI and extent of fibrosis) were assessed according to the Knodell’s scoring system[10].
LILMCs were isolated from liver biopsies as previously described by Bertoletti et al[11]. Biopsies were washed twice in RPMI 1640 medium to remove contaminated blood and digested with collagenase type-I (1 mg/mL CO130, Sigma Chemical Co., USA) and deoxyribonuclease I (25 mg/mL D4263, Sigma Chemical Co., USA) for 1 h at 37 °C on a shaking device. The liver specimen was then pipetted vigorously to disrupt the hepatic tissue and to release infiltrating lymphomononuclear cells. The mononuclear cell suspension was washed twice and the cells were recovered by centrifugation over a Ficoll-hypaque density gradient. Cells were then suspended in RPMI 1640 (GIBCO Lab) containing 10% heat inactivated fetal calf serum, 25 mmol/L HEPES, 2 mmol/L L-glutamine and 50 µg/mL gentamycin. Viability was >95% by the trypan blue dye exclusion test. Cells were cultured in duplicate (1.0×105 viable cells/100 µL) in flat-bottomed 96-well plates at 37 °C in an atmosphere containing 50 mL/L CO2 with medium alone (unstimulated culture) and stimulated with 10 µg/mL phytohemagglutinin (1:100 PHA, Sigma Chemical Co., USA) and recombinant IL-12 (20 U/mL, R&D Systems, USA). Restimulation was performed every 10 d. Supernatants were collected at the end of the proliferation assay and kept frozen at -70 °C for later analyses. Culture supernatants and sera were thawed and the serum level of cytokines was measured by ELISA.
The local ethic committee approved this study. Informed consent was obtained from each patient included in this study. Data were presented as mean±SD. Data analysis was made by the χ2, Fisher’s exact, Mann-Whitney U and Pearson’s correlation tests using SPSS for Windows (Chicago, IL, USA) when appropriate. P<0.05 was considered statistically significant.
Age, gender and viral load were similar in groups 1 and 2 (Table 1). The results of biochemical analyses except for aspartate aminotransferase (AST) and ALT were also similar in both the groups. In group 1, five patients underwent liver biopsy before being enrolled in the study. Though liver histology was similar in three patients, histological progression from stage 1 to stage 3 was observed in two of them.
| Normal ALT | Elevated ALT | P | |
| n | 16 | 17 | |
| Age | 51.4±10 | 47±10.7 | >0.05 |
| Female/male | 10/6 | 8/9 | >0.05 |
| Duration of infection (mo) | 33±32 (12-108) | 21±22 (6-72) | >0.05 |
| ALT (IU/L) | 36±6 | 115±60 | <0.05 |
| AST (IU/L) | 32±7.8 | 70±21 | <0.05 |
| ALP (IU/L) | 187.9±95 | 219.8±104.5 | >0.05 |
| GGT (IU/L) | 50.2±92.7 | 102.5±81.6 | >0.05 |
| Total bilirubin (mg/dL) | 0.7±0.5 | 0.6±0.2 | >0.05 |
| Albumin (g/dL) | 4.1±0.2 | 4±0.3 | >0.05 |
| Globulin (g/dL) | 1.2±0.2 | 1.4±0.4 | >0.05 |
| Histology | |||
| Minimal abnormalities | 3 | 0 | |
| Stage (1/2/3/4) | 9/1/3/0 | 010/5/0/2 | |
| HAI | 4.4±2.8 | 6.1±3.5 | >0.05 |
| HCV-RNA (copies/mL) | 456 532±277 761 | 461 710±324 860 | >0.05 |
Serum levels of IFN-γ (1.2±1.8; 1.1±0.9 pg/mL), IL-12 (0.2±0.4; 0.11±0.26 pg/mL), IL-10 (17.7±55.6; 18.2±47.8 pg/mL) and TNF-α (0.9±1.2; 0.4±0.25 pg/mL) were similar in groups 1 and 2 (P>0.05). Both stimulated IFN-γ and TNF-α levels in LILMCs were increased in comparison to their serum levels in both groups.
In ALT normal patients, there was a positive correlation between TNF-α and IFN-γ levels (both serum and liver) (P<0.05, r = 0.5). IL-12 and IL-10 levels stimulated by IL-2 were higher in group 1 than in group 2 (P<0.05, Table 2).
| pg/mL | Normal ALT | Elevated ALT | P |
| IFN-γ | |||
| IL-2 | 1.8±2.3 | 2.2±3.4 | >0.05 |
| PHA | 2.1±2.7 | 2.8±3.7 | >0.05 |
| IL-12 | |||
| IL-2 | 0.6±0.5 | 0.24±0.5 | 0.027 |
| PHA | 0.9±0.6 | 0.7±0.7 | >0.05 |
| IL-10 | |||
| IL-2 | 12.2±23.8 | 4.1±5.3 | 0.023 |
| PHA | 15.9±30.6 | 9.2±7.1 | >0.05 |
| TNF- | |||
| IL-2 | 2.5±1.06 | 2.5±2.9 | >0.05 |
| PHA | 3.3±1.4 | 3.8±3.2 | >0.05 |
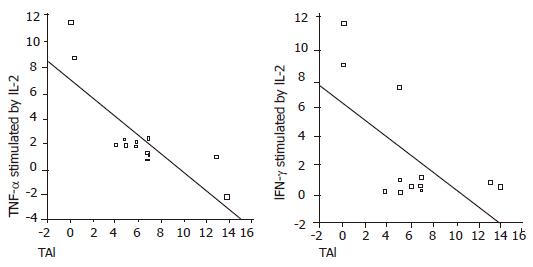
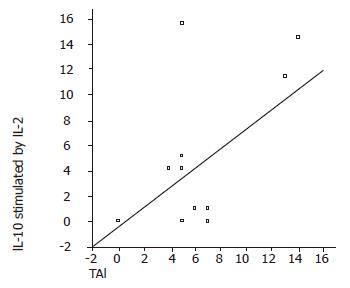
In elevated ALT patients, there was a negative correlation between serum IL-12 and IFN-γ levels (P = 0.01, r = –0.6). HAI and stage had a negative correlation with TNF-α (P<0.05, r = –0.8; P<0.05, r = –0.6, respectively) and IFN-γ (P<0.05, r = –0.5; P<0.05, r = –0.4, respectively) levels (Figure 1). IL-10 stimulated by IL-2 had a positive correlation with HAI (P<0.05, r = 0.49) and stage (P<0.05, r = 0.59) (Figure 2). TNF-α and IFN-γ levels in LILMCs were positively correlated (P<0.05, r = 0.8). Cytokine levels in serum and LILMCs were not correlated with serum HCV-RNA loads in both groups.
Factors involved in the progression to end-stage liver disease in HCV-infected patients are not well characterized. It is thought that cytotoxic T lymphocyte (CTL) response early in infection may be important for viral clearance, while continuous low-level anti-HCV CTL-dependent immune response may be responsible for accumulated liver damage[12]. Prezzi et al[13] showed that LILMCs have phenotypic and functional characteristics distinct from peripheral blood lymphocytes. All these immunological processes define natural progress of HCV infection. Patients with normal and elevated ALT levels show different clinical patterns[14]. Generally, HCV carriers with normal ALT have mild and stable diseases with a favorable prognosis[8]. Liver histology was normal in 20% of our cases with persistently normal ALT. Progression of liver fibrosis was observed in two of five patients (40%) who had a second liver biopsy in a period of 41.5±22.1 months.
Immunological studies concerning HCV infection generally focus on T lymphocytes. However, the serum cytokine levels have been found to be different in chronic hepatitis C patients[2,15,16]. Rico et al[1] showed that HCV specific CD4+ T-cell proliferation responses do not parallel in LILMCs and peripheral blood mononuclear cells. The magnitude of T-cell response is higher in the liver than in peripheral blood. In our study, serum levels of cytokines were similar in patients with persistently normal and elevated ALT, suggesting that liver cytokine levels are more important than serum levels in mediating T-cell responses.
IL-10 and IL-12 levels stimulated with IL-2 were higher in patients with normal ALT than in those with elevated ALT (P<0.05). While IL-10 showed Th2 response, IL-12 promoted Th1 cell induction and cell-mediated immunity. Interestingly both of them were high in patients with normal ALT, suggesting that strong Th2 response may be the cause of the mild biochemical and histological activity in patients with normal ALT.
Sobue et al[4] revealed that disease activity and progression correlate with dominant Th1 response in chronic hepatitis C patients. On the other hand, Tsai et al[17] showed that predominant Th1 response is stronger in patients with resolved infection than in those with chronic diseases. In our study, while histological stage and HAI were increased, TNF-α and IFN-γ levels were decreased in patients with elevated ALT (Figure 1). TNF-α and IFN-γ trigger antiviral defense mechanisms and have a principal effect on inflammation[6,7]. This means that the magnitude of antiviral immune response is decreased, while the histological activity and stage are increased. Though Th1 and Th2 responses were both strong in patients with normal ALT, Th1 response was not as high as that in patients with elevated ALT, suggesting that strong antiviral defense against HCV infection can normalize liver enzymes. On the other hand, histological abnormalities might be impaired by the increased Th2 response.
In conclusion, both Th1 and Th2 responses in liver are elevated in patients with normal ALT. Increased Th2 response may regress inflammatory activity. In patients with normal ALT, progression of histological findings may probably depend on insufficient Th2 response, which does not balance Th1 response. A differentiation between virus-specific and non-specific T-cell populations is the greatest challenge in future studies.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Rico MA, Quiroga JA, Subira D, Garcia E, Castanon S, Sallberg M, Leroux-Roels G, Weiland O, Pardo M, Carreno V. Features of the CD4 T-cell response in liver and peripheral blood of hepatitis C virus-infected patients with persistently normal and abnormal alanine aminotransferase levels. J Hepatol. 2002;36:408-416 DOI : 10.1016/S0168-8278(01)00281-1. |
| 2. | Cacciarelli TV, Martinez OM, Gish RG, Villanueva JC, Krams SM. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alfa. Hepatology. 1996;24:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 177] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Koziel MJ. Cytokines in viral hepatitis. Semin Liver Dis. 1999;19:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Sobue S, Nomura T, Ishikawa T, Ito S, Saso K, Ohara H, Joh T, Itoh M, Kakumu S. Th1/Th2 cytokine profiles and their relationship to clinical features in patients with chronic hepatitis C virus infection. J Gastroenterol. 2001;36:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, Eichenlaub D, Pape GR. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 522] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | Neuman MG, Benhamou JP, Malkiewicz IM, Ibrahim A, Valla DC, Martinot-Peignoux M, Asselah T, Bourliere M, Katz GG, Shear NH. Kinetics of serum cytokines reflect changes in the severity of chronic hepatitis C presenting minimal fibrosis. J Viral Hepat. 2002;9:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Frese M, Barth K, Kaul A, Lohmann V, Schwärzle V, Bartenschlager R. Hepatitis C virus RNA replication is resistant to tumour necrosis factor-alpha. J Gen Virol. 2003;84:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Puoti C. HCV carriers with persistently normal aminotransferase levels: normal does not always mean healthy. J Hepatol. 2003;38:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Mathurin P, Moussalli J, Cadranel JF, Thibault V, Charlotte F, Dumouchel P, Cazier A, Huraux JM, Devergie B, Vidaud M. Slow progression rate of fibrosis in hepatitis C virus patients with persistently normal alanine transaminase activity. Hepatology. 1998;27:868-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis [Hepatology 1981; 1: 431-435]. J Hepatol. 2003;38:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2558] [Cited by in RCA: 2507] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 11. | Bertoletti A, D'Elios MM, Boni C, De Carli M, Zignego AL, Durazzo M, Missale G, Penna A, Fiaccadori F, Del Prete G. Different cytokine profiles of intraphepatic T cells in chronic hepatitis B and hepatitis C virus infections. Gastroenterology. 1997;112:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 225] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003;38:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Prezzi C, Casciaro MA, Francavilla V, Schiaffella E, Finocchi L, Chircu LV, Bruno G, Sette A, Abrignani S, Barnaba V. Virus-specific CD8(+) T cells with type 1 or type 2 cytokine profile are related to different disease activity in chronic hepatitis C virus infection. Eur J Immunol. 2001;31:894-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Jamal MM, Soni A, Quinn PG, Wheeler DE, Arora S, Johnston DE. Clinical features of hepatitis C-infected patients with persistently normal alanine transaminase levels in the Southwestern United States. Hepatology. 1999;30:1307-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Osna N, Silonova G, Vilgert N, Hagina E, Kuse V, Giedraitis V, Zvirbliene A, Mauricas M, Sochnev A. Chronic hepatitis C: T-helper1/T-helper2 imbalance could cause virus persistence in peripheral blood. Scand J Clin Lab Invest. 1997;57:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kallinowski B, Haseroth K, Marinos G, Hanck C, Stremmel W, Theilmann L, Singer MV, Rossol S. Induction of tumour necrosis factor (TNF) receptor type p55 and p75 in patients with chronic hepatitis C virus (HCV) infection. Clin Exp Immunol. 1998;111:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Tsai SL, Liaw YF, Chen MH, Huang CY, Kuo GC. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology. 1997;25:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 274] [Article Influence: 9.8] [Reference Citation Analysis (0)] |