Published online Dec 7, 2005. doi: 10.3748/wjg.v11.i45.7104
Revised: June 2, 2005
Accepted: June 6, 2005
Published online: December 7, 2005
AIM: To transfect mutant C-kit cDNA at codon 579 into human embryonic kidney cell line to observe its role in the pathogenesis of gastrointestinal stromal tumor (GIST).
METHODS: Eukaryotic expression vectors of pcDNA3-Kit-NW and pcDNA3-Kit-W were constructed. Then pcDNA3-Kit-NW and pcDNA3-Kit-W plasmids were transfected into human embryonic kidney cell line by Lipofectamine. The resistant clone was screened by G418 filtration and identified by sequencing, Western blotting, and immunocytochemical staining. Human embryonic kidney cells were divided into three groups including pcDNA3-Kit-NW, pcDNA3-Kit-W, and vector control groups. Absorbency value with a wavelength of 574 nm was detected by MTT analysis. Mice were injected with three groups of cells. Volume, mass, and histological examinations of the tumors in different groups were measured and compared.
RESULTS: The C-kit gene and mutant C-kit gene were successfully cloned into the eukaryotic expression vector pcDNA3. pcDNA3-Kit-NW and pcDNA3-Kit-W were successfully transfected into human embryonic kidney cell line and showed stable expression in this cell line. Cell proliferating activity had significant differences between pcDNA3-Kit-NW and pcDNA3, pcDNA3-Kit-NW and pcDNA3-Kit-W (P<0.05), respectively. Tumors were only observed in nude mice implanted with cells transfected with pcDNA3-Kit-NW.
CONCLUSION: Mutation of C-kit gene increases the proliferation activity of human cells and plays an important role in the malignant transformation of GIST.
- Citation: Bai CG, Liu XH, Xie Q, Feng F, Ma DL. A novel gain of function mutant in C-kit gene and its tumorigenesis in nude mice. World J Gastroenterol 2005; 11(45): 7104-7108
- URL: https://www.wjgnet.com/1007-9327/full/v11/i45/7104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i45.7104
Gastrointestinal stromal tumor (GIST) is a designation for a major subset of mesenchymal tumors of the gastrointestinal tract. Their line of differentiation or cell of origin and clinical behavior are a persistent source of controversy[1-5]. Recently, specific mutations between trans-membrane and tyrosine kinase domains in exon 11 at codon 550-560 of C-kit have been found in GISTs, which can lead to ligand-independent activation of the tyrosine kinase of C-kit and have a tumor promoting effect in vitro[4,6-10]. We studied the C-kit mutation type of exon 11 by PCR-SSCP and DNA sequencing in a series of Chinese GIST[11]. The results showed that C-kit mutations existed among GISTs. Compared to the reports in the published data, an insertion of 12 bp at codon 579 lies outside the hot spot area[12]. In order to fully characterize the activation of the insert mutation at codon 579 of C-kit gene, we constructed an expression vector containing mutant C-kit and evaluated its effect on implanted tumor in nude mice.
Fetal bovine serum was produced by Hyclone and 96-well plates by Costa. Dimethyl sulfoxide (DMSO), ethylenediaminetetraacetic acid (EDTA), 3-(4,5-dimethy1-thiazol-2-yl), 2,5-diphenyl tetrazolium bromide (MTT), N-2-hydroxyethyl piperazine-N’-2-ethanesulfonic acid (HEPES) and trypsin were the products of Sigma. Rabbit polyclonal antibody against C-kit was obtained from DAKO. Envision two-step and DAB test kits were obtained from Beijing Zhongshan Biotechnology Inc.
Human embryonic kidney cell (HEKC) line was established in our laboratory and prepared after long-term generation.
Eukaryotic expression vector of pcDNA3 (Invitrogen, USA), plasmid pMD18-T-KIT-NW containing full-length of C-kit gene (C-kit cDNA was conducted in our laboratory) and plasmid pMD18-T-Kit-NW containing mutant-type C-kit cDNA with insertion of 12 bp at codon 579 (mutant C-kit cDNA was conducted in our laboratory) were digested by restriction enzymes XbaI and HindIII. The digested vector and plasmid cDNA fragment were ligated. Recombinant clones were identified by XbaI and HindIII double digestion. Positive clones named pcDNA3-Kit-W and pcDNA3-Kit-NW were further confirmed by sequencing.
Cultured HEKCs were divided into three groups, which were transfected with pcDNA3 vector, recombinant pcDNA3-Kit-W, and recombinant pcDNA3-Kit-NW, respectively. Transfection was performed according to the instructions of Lipofectamine TM2000 reagent kit (Gibco). Cells were cultured in RPMI 1640 medium containing 200 mL/L fetal calf serum and 350 mg/L G418. Resistant clones could be detected 2 wk later.
HEKCs were inoculated in RPMI 1640 with 100 g/L fetal bovine serum and incubated at 37 °C in an incubator containing 50 mL/L CO2 to measure the logarithmic growth phase. After the treatment with digestive fluid, HEKCs were suspended by adding D-Hank’s fluid, deposited by centrifugation at 190 r/min for 5 min and then counted. Using RPMI 1640 containing 100 g/L fetal bovine serum, HEKCs were adjusted to a density of 5×104/mL and added to a 24-well plate containing flying sheets, 0.2 mL/well. Flying sheets were taken out respectively at 24, 48, and 72 h, fixed with cold acetone for 10 min, dried and preserved at -60 °C.
Preserved cell flying sheets were immersed in PBS for 5 min, blocked with 10 mL/L H2O2 for 10 min, washed thrice with PBS (5 min each time) and then incubated with 10% goat serum for 30 min. C-kit antibody was diluted at a concentration of 1:50 and added as the first antibodies, staying 1 h at 37 °C. After being washed thrice with PBS (5 min each time), horseradish peroxidase-conjugated goat anti-rabbit secondary antibody was added, staying 30 min at 37 °C, washed thrice with PBS (5 min each time) and stained with DAB. Counterstaining with hematoxylin was performed before the final analysis. The first antibody was replaced with fetal bovine serum as a negative control.
Cell lysates were prepared in extraction buffer containing 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 10 g/L Triton-100, 1 g/L SDS, 1 mmol/L EDTA, 1 mmol/L AEBSF, 20 g/mL aprotinin, and 20 g/mL leupeptin. After centrifugation at 12 000 g for 10 min at 4 °C, the supernatant was collected. Equal amounts of total protein (10 g) from each sample were loaded and separated by 120 g/L SDS-polyacrylamide gel electrophoresis and then transferred to Hybond-P polyvinylidene difluoride (PVDF) membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). After being blocked with 50 g/L nonfat dry milk in PBS (pH 7.4) with 1 g/L Tween-20, membranes were probed with a rabbit anti-C-kit polyclonal antibody (1:500 dilution) followed by subsequent incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1 000 dilution).
HEKCs with a density of approximately 2.5×105/mL were seeded into each well of the 96-well plate (0.2 mL/well). Each group contained 12 wells. After 4 d of incubation at 37 °C in an incubator containing 50 mL/L CO2, 50 mL of MTT (50 mg MTT) was added and the culture was continued for 4 h at 37 °C. After the fluid at the top was removed, 150 mL DMSO was added to each well. After being concussed and dissolved, absorbency value with a wavelength of 574 nm was detected by enzyme-labeled instrument (BioRad 2250, Japan).
Nine Balb/c male mice aged 4-6 wk (body mass 18-20 g) were purchased from Experimental Animal Center of Second Military Medical University, Shanghai and randomly divided into three groups, three mice in each group. The mice in vector-treated control group were injected with HEKCs transfected with pcDNA3 vector, the mice in Kit-W group received an injection of HEKCs transfected with recombinant pcDNA3-Kit-W, the mice in Kit-NW group received an injection of HEKCs transfected with recombinant pcDNA3-Kit-NW. After the cancer cells were cultured into the stage of logarithmic growth phase, they were digested with trypsin to make cancer cell suspension of 2.5×107/L. Then, 0.2 mL of each suspension was subcutaneously injected into the right back of the nude mice.
The survival of nude mice was observed every day. After tumor cell injection, the nude mice were killed after 6 wk. The surrounding fatty tissues were dissected and the tumors were weighed. Tumors were fixed in 10% buffered formalin and processed in paraffin wax. Five-micrometer thick sections were stained with hematoxylin and eosin.
Data were treated with SPSS for LSD and one-way ANOVA test. P<0.05 was considered statistically significant.
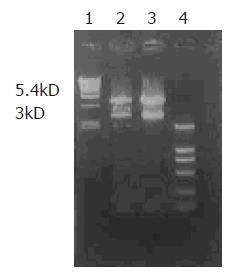
After digestion by XbaI and HindIII, bands at 3 kD could be detected for positive clones (Figure 1), suggesting that Kit-W and Kit-NW fragments were inserted into the pcDNA3 vector, named as recombinant plasmids pcDNA3-Kit-W and pcDNA3-Kit-NW, respectively.
pcDNA3-Kit-W and pcDNA3-Kit-NW DNA were prepared for sequencing. The sequence obtained was the same as the reported sequence of C-kit cDNA and mutant C-kit cDNA, indicating that the C-kit gene and mutant C-kit gene were successfully cloned into the eukaryotic expression vector pcDNA3.
No significant differences were observed between the morphological characteristics of transfected cells and normal HEKCs.
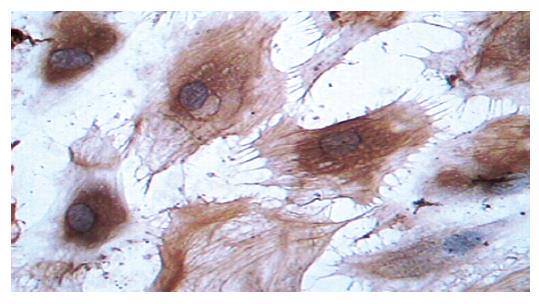
Supernatants of cultured cells in three groups were collected and analyzed by immunocytochemistry. The cells transfected with pcDNA3-Kit-W and pcDNA3-Kit-NW were positively stained for C-kit protein (Figure 2). However, the cells in vector control group were negatively stained for C-kit. In accordance with the findings on immunocytochemical staining, a band in a molecular mass of 145 ku was detected with rabbit anti-C-kit antibody from the cells transfected with pcDNA3-Kit-W and pcDNA3-Kit-NW, but no bands were detected in the vector control group. There was no significant difference in the protein level of C-kit between pcDNA3-Kit-W and pcDNA3-Kit-NW groups (Figure 3).
Absorbance values of the three groups were 0.1340±2.353×10-3, 0.1830±3.888×10-3, and 0.1274±3.537×10-3, respectively. LSD analysis and one-way ANOVA analysis with SPSS10.0 software were used to compare the difference of absorbance in each group at 574 nm. Statistical analysis indicated that significant differences were detected between pcDNA3-Kit-NW and pcDNA3, pcDNA3-Kit-NW and pcDNA3-Kit-W (P<0.05), respectively. The results implied that the cell proliferating activity in pcDNA3-Kit-NW group was higher than that in control group.
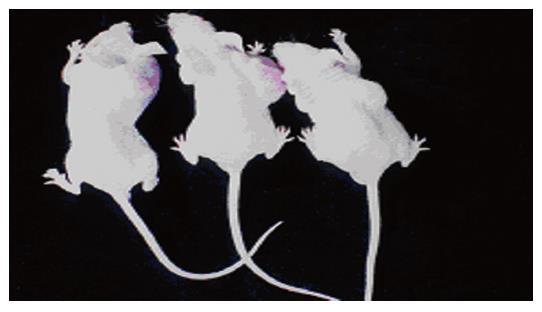
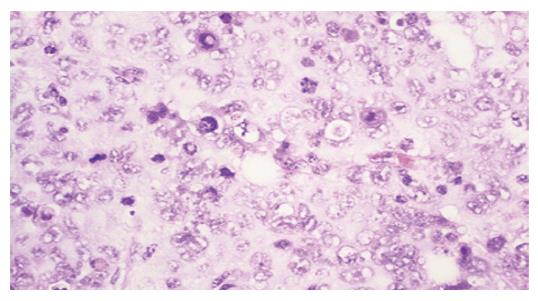
Tumors were observed in the nude mice 3 wk after being implanted with cells transfected with pcDNA3-Kit-NW (Figure 4). Mice had no visible tumor after cell injection in pcDNA3-Kit-W transfection group and vector control group after 6 wk. Tumor volumes in pcDNA3-Kit-NW transfection group were approximately similar and no significant difference was observed between the three tumors (t = 13.07 P>0.05). Many areas of hemorrhage and necrosis were present, residual tumor cells were frequently found to have giant, bizarre-shaped pyknotic nucleoli, or prominent pathologic mitosis in the tumors (Figure 5).
GIST is the most common mesenchymal tumor in human gastrointestinal tract and is enigmatic in terms of its differentiation or cell origin and clinical behavior. In recent years, C-kit protein expression and activated gene mutation have been found in GIST[13-17]. C-kit encodes a growth factor receptor with ligand-dependent tyrosine kinase activity[18,19]. Kit ligand-stem cell factor (SCF) is the only known ligand of the Kit receptor[20]. SCF binding to the receptor mediates receptor dimerization, activation of kinase activity, and autophosphorylation. Subsequently, Kit activates several signaling cascades, leading to cell proliferation, cell survival, and other cellular responses. Kit and SCF are encoded at the white spotting (W) and steel (Sl) loci in mice, respectively[20-22]. Mutation at the murine W and Sl loci generates deficiencies in several major cell systems during embryogenesis and in postnatal animals. In hematopoiesis Kit receptor signaling is critical in the stem cell hierarchy, erythropoiesis, mast cell development and function, megakaryopoiesis, and lymphopoiesis[23-26].
GISTs most often harbor mutations at exon 11 codon 550-560 of C-kit cDNA, while the mutations in human mastocytosis predominantly involve the activation loop of the Kit kinase[27,28]. The C-kit mutations in this domain can lead to spontaneous ligand-independent activation of the tyrosine kinase of C-kit and was called "gain-of-function mutation". Development of GIST is highly associated with Kit-activating mutations, suggesting that the activated Kit receptor plays a critical role in tumor development[29]. In addition, gene mutation has shown a good correlation with biologic behavior in such tumors. For instance, it can provide valuable adjunctive prognostic information. In agreement with this notion, familial cases of GIST have been reported with Kit-activating mutations in the germ line[30]. We have found a mutation at codon 579 in malignant GIST, which lies outside the hot spot area[12]. In order to fully characterize the activation of the insert mutation at codon 579 of C-kit gene, we studied the expression of mutant C-kit gene in human embryonic kidney cell line and its effect on implanted carcinoma in the nude mice.
In the present study, we constructed a target fragment containing mutant-type C-kit cDNA with insertion of 12 bp at codon 579 and full-length of C-kit gene. The target gene fragment of 5.4 kD in length was cloned into XbaI and HindIII restriction sites of eukaryotic expression vector pcDNA3. Restriction digestion and sequence analysis for positive clones indicated that the two recombinant eukaryotic expression vectors containing the gene were successfully constructed. These recombinant vectors were then transfected into HEKCs and screened by G418. The result of direct sequencing showed that the recombinant plasmids were stably integrated into the cells. Immunocytochemical staining revealed that HEKCs transfected with pcDNA3-Kit-W and pcDNA3-Kit-NW were stained positively for C-kit, indicating that C-kit protein is expressed in transfected HEKCs. Furthermore, a protein (145 kD) was detected by Western blotting and there was no significant difference in the expression level of C-kit protein between transfected pcDNA3-Kit-W and pcDNA3-Kit-NW HEKCs, indicating that the insert mutation at codon 579 of C-kit gene has no effect on protein expression of HEKCs.
In vitro characterization of the Kit V558 mutation in BaF/3 cells indicates that the Kit V558 mutation promotes cell proliferation and abolishes the growth factor dependence of BaF/3 cells[30]. Sommer et al[31]. showed that young bone marrow-derived mast cell cultures are refractory to growth factor deprivation-induced apoptosis. However, these cultures do not promote cell cycle progression. There is evidence that short kit protein product is more active than long Kit protein product. Therefore, it may be possible that the long-Kit variant is expressed predominantly in the young mutant bone marrow-derived mast cells, while the older cultures express the short-Kit variant, which may explain the progression from partial to complete growth factor independently of these cultures. Our results showed that the insert mutation at codon 579 of C-kit gene promoted HEKC proliferation. In addition, no significant differences between the morphological characteristics of transfected cells and normal HEKCs were observed, suggesting that mutant C-kit has no effect on the morphology of HEKCs.
In this experiment, HEKCs transfected with recombinant pcDNA3-Kit-NW and pcDNA3 vector were subcutaneously implanted into the nude mice. The implanted tumors appeared later in the pcDNA3-Kit-NW transfection group. Mice had no visible tumor after cell injection in pcDNA3-Kit-W transfection group and vector control group after 6 wk. These results suggest that the insert mutation at codon 579 of C-kit gene can significantly induce the growth of tumors and constitutive Kit signaling is critical and sufficient for the induction of neoplasia in the mice.
Co-first-authors: Chen-Guang Bai and Xiao-Hong Liu
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Wong NA, Young R, Malcomson RD, Nayar AG, Jamieson LA, Save VE, Carey FA, Brewster DH, Han C, Al-Nafussi A. Prognostic indicators for gastrointestinal stromal tumours: a clinicopathological and immunohistochemical study of 108 resected cases of the stomach. Histopathology. 2003;43:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Nagasako Y, Misawa K, Kohashi S, Hasegawa K, Okawa Y, Sano H, Takada A, Sato H. Evaluation of malignancy using Ki-67 labeling index for gastric stromal tumor. Gastric Cancer. 2003;6:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Lin SC, Huang MJ, Zeng CY, Wang TI, Liu ZL, Shiay RK. Clinical manifestations and prognostic factors in patients with gastrointestinal stromal tumors. World J Gastroenterol. 2003;9:2809-2812. [PubMed] |
| 4. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 866] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 5. | Kim MK, Lee JK, Park ET, Lee SH, Seol SY, Chung JM, Kang MS, Yoon HK. [Gastrointestinal stromal tumors: clinical, pathologic features and effectiveness of new diagnostic criteria]. Korean J Gastroenterol. 2004;43:341-348. [PubMed] |
| 6. | Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res. 1999;59:4297-4300. [PubMed] |
| 7. | Berman J, O'Leary TJ. Gastrointestinal stromal tumor workshop. Hum Pathol. 2001;32:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 384] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, Sobin LH, Miettinen M. Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. A study of 200 cases. Am J Pathol. 2000;157:1091-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 11. | Ma D, Liu X, Cai Z, Xie Q. Expression and mutation of c-kit gene in gastrointestinal stromal tumor. Zhonghua Zhong Liu Za Zhi. 2002;24:461-464. [PubMed] |
| 12. | Feng F, Liu XH, Xie Q, Liu WQ, Bai CG, Ma DL. Expression and mutation of c-kit gene in gastrointestinal stromal tumors. World J Gastroenterol. 2003;9:2548-2551. [PubMed] |
| 13. | Hou Y, Wang J, Zhu X, Du X, Sun M, Zheng A. A clinicopathologic and immunohistochemical study on 76 cases of gastrointestinal stromal tumors. Zhonghua BingLiXue ZaZhi. 2002;31:20-25. [PubMed] |
| 14. | Liu XH, Ma DL, Xie Q, Wu LL. Stromal tumors of the duodenum: a clinicopathological and immunohistochemical study of 18 cases. Linchuang Yu Shiyan Binglixue Zazhi. 2002;18:122-126. |
| 15. | Ma DL, Liu XH, Bai CG, Xie Q, Feng F. Effect of c-kit gene mutation on prognosis of gastrointestinal stromal tumor. Zhonghua WaiKe ZaZhi. 2004;42:140-144. [PubMed] |
| 16. | Ma DL, Liu XH, Bai CG, Wu LL, Xie Q. Stromal tumors of the esophagus: a clinicopathological and immunohistochemical study. Zhonghua Waikexue Zazhi. 2002;40:237. |
| 17. | Liu XH, Ma DL, Bai CG, Wu LL, Xie Q. Gastrointestinal primary stromal tumor in omentum and mesentery: a clinicopathological and immunohistochemical study. Jiefangjun Yixue Zazhi. 2002;5:401-402. |
| 18. | Besmer P, Murphy JE, George PC, Qiu FH, Bergold PJ, Lederman L, Snyder HW, Brodeur D, Zuckerman EE, Hardy WD. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986;320:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 429] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Qiu FH, Ray P, Brown K, Barker PE, Jhanwar S, Ruddle FH, Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988;7:1003-1011. [PubMed] |
| 20. | Besmer P. The kit ligand encoded at the murine Steel locus: a pleiotropic growth and differentiation factor. Curr Opin Cell Biol. 1991;3:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 125] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 932] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 22. | Geissler EN, Cheng SV, Gusella JF, Housman DE. Genetic analysis of the dominant white-spotting (W) region on mouse chromosome 5: identification of cloned DNA markers near W. Proc Natl Acad Sci USA. 1988;85:9635-9639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 662] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 24. | Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova RF. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl. 1993;125-137. [PubMed] |
| 25. | Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 272] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Besmer P. Kit-ligand-Stem Cell Factor. Colony Stimulating Factors, Molecular& Cell Biology, Second Edition. New York: Marcel Dekker 1997; 369-404. |
| 27. | Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA. 1995;92:10560-10564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 657] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 28. | Brockow K, Metcalfe DD. Mastocytosis. Curr Opin Allergy Clin Immunol. 2001;1:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Kissel H, Timokhina I, Hardy MP, Rothschild G, Tajima Y, Soares V, Angeles M, Whitlow SR, Manova K, Besmer P. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000;19:1312-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 266] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Nishida T, Hirota S, Taniguchi M, Hashimoto K, Isozaki K, Nakamura H, Kanakura Y, Tanaka T, Takabayashi A, Matsuda H. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet. 1998;19:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 388] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 31. | Sommer G, Agosti V, Ehlers I, Rossi F, Corbacioglu S, Farkas J, Moore M, Manova K, Antonescu CR, Besmer P. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci USA. 2003;100:6706-6711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |