Published online Dec 7, 2005. doi: 10.3748/wjg.v11.i45.7084
Revised: July 23, 2005
Accepted: July 30, 2005
Published online: December 7, 2005
AIM: To investigate the anti-ischemic properties of perfluorochemical emulsion "perftoran" in mesenteric region.
METHODS: Experiments were conducted on 146 nonlinear white male rats weighing 200-350 g. Partial critical intestinal ischemia was induced by thorough atraumatic strangulation of 5-6 cm jejunal loop with its mesentery for 90 min. Global critical intestinal ischemia was made by atraumatic occlusion of the cranial mesenteric artery (CMA) for 90 min also. Perftoran (PF, 0.8-1.0 mL per 100 g) in experimental groups or 0.9% sodium chloride in control groups was injected at 75 min of ischemic period. Mean systemic arterial blood pressure (BPM) registration, intravital microscopy and morphological examination of ischemic intestine and its mesentery were performed in both groups.
RESULTS: During 90 min of reperfusion, BPM progressively decreased to 27.3±7.4% after PF administration vs 38.6±8.0% in the control group of rats with partial intestinal ischemia (NS) and to 50.3±6.9% vs 53.1±5.8% in rats after global ischemia (NS). During the reperfusion period, full restoration of microcirculation was never registered; parts with restored blood flow had leukocyte and erythrocyte stasis and intra-vascular clotting, a typical "non-reflow" phenomenon. The reduction of mesenteric 50-400 μm feeding artery diameter was significantly less in the PF group than in the control group (24±5.5% vs 45.2±3.6%, P<0.05) 5 min after partial intestinal ischemia. This decrease progressed but differences between groups minimized at the 90th min of reperfusion (41.5±4.2% and 50.3±2.8%, respectively). In reperfusion of rat's intestine, a significant mucosal alteration was registered. Villous height decreased 2.5-3 times and the quantity of crypts decreased more than twice. In the group of rats administered PF, intestinal mucosal layer was protected from irreversible post-ischemic derangement during reperfusion. Saved cryptal epithelial cells were the source of regeneration of the epithelium, which began to cover renewing intestinal villi after 24 h of blood flow restoration. View of morphological alterations was more heterogeneous in CMA groups.
CONCLUSION: Systemic administration of perftoran promotes earlier and more complete structural regeneration during reperfusion in rats after partial and global critical intestinal ischemia.
- Citation: Kozhura VL, Basarab DA, Timkina MI, Golubev AM, Reshetnyak VI, Moroz VV. Reperfusion injury after critical intestinal ischemia and its correction with perfluorochemical emulsion "perftoran". World J Gastroenterol 2005; 11(45): 7084-7090
- URL: https://www.wjgnet.com/1007-9327/full/v11/i45/7084.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i45.7084
Prophylaxis and treatment of ischemia-reperfusion injuries in common and especially in mesenteric circulation are the most actual problems of modern-day surgery, intensive care medicine and pathology, and still require improved understanding of the pathophysiology and mechanisms of the development of these disorders[1,2]. Pathophysiological derangement, which occurs after restoration of a blood flow known as the reperfusion syndrome, continues to be responsible for the very high mortality rate in the fields of surgery associated with acute mesenteric occlusion or intestinal strangulation[3,4]. There is no generally accepted opinion about the critical terms of intestinal ischemia. During this time, the answer to this question has not only theoretical but also important practical significance. For example, this problem is one of the main aspects in the field of intestinal transplantation[5,6].
There has been extraordinary progress in the treatment of ischemia-reperfusion injuries over the past two decades. Though advances have been made in the management of acute vascular occlusion, the optimal treatment of a reperfusion injury is still not defined. Perfluorocarbon emulsion (PFCE) is among the medicines administered in different cases of ischemia-reperfusion injuries. Investigations in this field are one of the most priority branches of scientific researches in the world. Designation and intensive investigation of the effects of PFCE in different fields of biology and medicine have been conducted by a number of Russian scientists since 1979 under the general direction of the Institute of Biological Physics of the Acad. Sci. USSR. Perftoran (OAO NPF "Perftoran", Puschino Russia) is a well-known PFCE containing 20% of perfluorodecalin and perfluoromethylcyclohexylpiperidin stabilized by proxanol-168. The diameter of an average particle is about 0.07 μm. Local (intraluminal or intraperitoneal) administration of PFCE on intestinal ischemia-reperfusion injury has been extensively elucidated[7-13].
All animal procedures were performed in accordance with the recommendations for the proper use and care of laboratory animals. Experiments were performed on 146 white nonlinear adult male pathogen-free rats weighing 200-350 g. Before the surgery, they were anesthetized with pentobarbital sodium (CPF, Tallinn, Estonia) (5-7 mg per 100 g, i m). Except for chronic experiments, tracheostomy cannula was inserted and animals were supported by time-cycled, volume-limited ventilation with room air by mechanical respirator 4601-1 Advanced (Technical and Scientific Equipment, GmbH, Germany) at the frequency of 70 breaths/min and the tidal volume of 5 mL/kg.
The rats were then randomized into two groups depending on the type of chosen critical intestinal ischemia. Each group was divided into two subgroups. In the subgroup A, perftoran was used as a protective remedy before reperfusion. In the subgroup B, the same volume of 0.9% sodium chloride was injected as a control.
In group 1 (n = 110), animals were exposed to a critical segmentary (local) tourniquet ischemia of the intestine. This model corresponded to incarcerated hernia or different types of bowel strangulation such as commissural ileus, volvulus, formation of knots and others. After a midline abdominal incision was made, intestinal ischemia was produced by atraumatic strangulation of the jejunal loop (5-6 cm in length) and its mesentery (15-30 cm distal after duodeno-jejunal flexion) with thick tourniquet until visual absence of regional artery pulsation for 90 min. In subgroup 1A (n = 48), perftoran (1 mL per 100 g) was injected slowly intra-arterially (i a) at 75 min of ischemic period. In subgroup IB (control group, n = 62), 0.9% sodium chloride solution was injected i.a. at the same time and volume.
In group 2 (n = 36), rats were exposed to a critical global (total) ischemia of the intestine. This model corresponded to acu 2A (n = 22), PF (1 mL per 100 g, 5-6% of circulating blood volume) was injected i a at 75 min of ischemic period. In subgroup 2B (control group, n = 14), 0.9% sodium chloride solution was injected i.a. at the same time and volume.
External hemoexfusion volume was thoroughly counted during the experiments and 0.95% sodium chloride (2:1) was injected i.a. in order to fill up the blood loss and maintain the volume of circulating plasma. In chronic experiments (n = 17), gut contents were replaced in the abdominal cavity, the incision was closed with suture technique and animals were returned to the prone position.
Caudal or femoral arteries were prepared and cannulated by polyethylene (PE)-10 or PE-50 catheters (Becton Dickinson, USA) after i.a. heparin administration (30 ME per 100 g). During the experiments, mean systemic arterial blood pressure (BPM) was monitored by pressure transducers of Bentley Trantec and Statham P231D (Cobe Laboratories, Inc., USA) or MPU-05-290-0-III (San-EI, Japan) connected to the polygraph Schwarzer (Picker International GmBH, Munchen, Germany) or the automatic recorder 142-8 (San-EI, Japan).
After a midline abdominal incision was made, the chosen segment of the proximal jejunum with mesentery was extracted on a transparent table of the microscope and covered by a transparent film to prevent tissue withering. Rectal temperature of the animal and intestinal loop was kept at 36.5 °C. Rat's mesenteric microcirculatory bed was observed under a through-passage light bio-microscope MBI-15 (LOMO, Russia) supplied with photo-camera Zenit-ET (Zenit, Russia), an original device for registration of micro-vessel diameters by the splitting image method[14]. An analogous signal with amplitude proportional to the external vessel diameter was registered simultaneously with BPM line on the automatic recorder 142-8. Diameters of the mesenteric micro-vessels were registered before ischemia and during 90 min of reperfusion.
Tissue samples were harvested from ischemic zone in experiments with tourniquet intestinal loop strangulation. In experiments with mesenteric occlusion, a very heterogeneous view of the injured intestine was registered. Morphological examination of the different segments of the intestinal wall was performed. Morphological samples were harvested just before the end of 1.5 ischemic period and after 1.5 and 24 h of reperfusion.
Tissue samples of the intestinal wall were prepared as rings, fixed in 10% neutral buffered formalin and embedded in paraffin. Four-to six-micrometer-thick sections were stained with hematoxylin and eosin and examined under light microscope. PAS-reaction was also conducted. Morphological examination was performed on 243 sections of the rat’s intestine by the "blind" method. The numerical six-step scale proposed in 1970 by Chiu et al[15]. was used for qualitative evaluation of the degree of the mucosal layer alterations.
Data were analyzed with "Microsoft Excel 97 SR-2" statistical software. All values were expressed as mean±SD. Paired and unpaired t-criteria were used to test the differences within and between groups, respectively. P<0.05 was considered statistically significant.
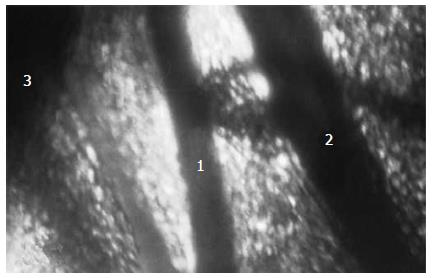
Tourniquet application on the intestinal loop (group I, n = 42) led to complete blood flow interruption in the mesenteric bed and intestinal wall. Erythrocyte diapedesis around the mesenteric micro-vessels increased with terms of ischemia. Capillary network structure underwent significant alterations. One minute after tourniquet removal, blood flow was registered visually as a rule, only in the large mesenteric vessels and their branches directly before the intestinal wall, the so-called "feeding" arteries and veins with a diameter from 100 to 400 μm (Figure 1). In smaller vessels, full restoration of microcirculation was never registered; parts with restored blood flow had leukocyte and erythrocyte stasis and intra-vascular clotting, a typical "no-reflow" phenomenon. Hemorrhages occupied considerable areas of intestinal mesentery. During the reperfusion period, decreased blood flow velocity, increased number of leukocytes with activated aggregation and leukocyte-endothelial wall interactions were also registered in the micro-vessels.
During the initial phase of global intestinal ischemia (group 2, n = 9) in some large mesenteric arteries, no total stasis but slow blood flow was registered in contrast with total mesenteric occlusion. Probably, total mesenteric occlusion activated the collateral circulation of ischemic intestine in the first few minutes. After 15-20 min of ischemia, full stasis came in the mesenteric bed. After the removal of occluder from CMA, blood flow was restored in large arteries and veins which divides mesentery on transparent "windows" and in branches near the intestinal wall, the "feeding" vessels. In smaller blood vessels of mesentery, the "no-reflow" phenomenon progressed within 90 min of reperfusion.
In general, reperfusion injuries of mesenteric microcirculation did not change either after partial tourniquet ischemia or CMA occlusion, but impairments were more heterogeneous in rats of group 2.
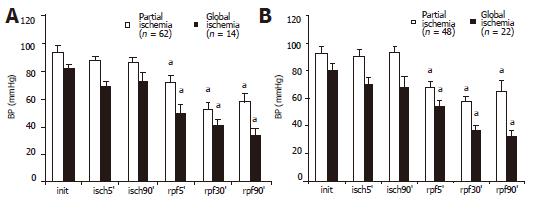
Systemic BPM level during 90 min of partial and global intestinal ischemia and reperfusion is shown in Figure 2A. BPM level did not change during the ischemic period but abrupt decrease was significant at 5 min of reperfusion (about 40% in cases with CMA occlusion). This systemic BPM level decrease continued till the end of the observation period (90 min of reperfusion).
Administration of PF in subgroups 1A and 2A (partial and global intestinal ischemia) had no impact on the general picture of microcirculatory alterations in the intestinal mesentery during reperfusion. As in the control group, the systemic BPM level progressively decreased in the post-ischemic period (Figure 2B). As shown in Figures 2A and B, changes in the BPM level during reperfusion had no differences after the administration of PF before the end of the ischemic period and in control rats. There were no significant differences between subgroups 1A and B or between subgroups 2A and B.
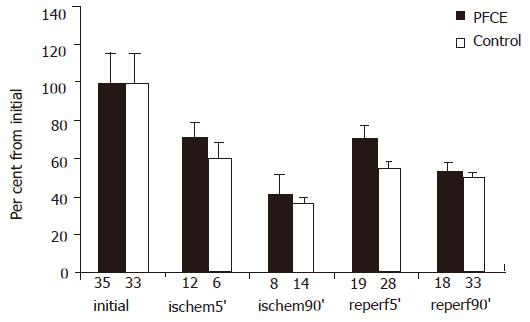
Though there were no post-ischemic significant changes in microcirculation of rat's intestinal mesenteric bed, intestinal blood supply was restored. During this time, blood flow restoration was not complete because the diameters of the "feeding" arteries in reperfusion period were significantly smaller. In eight rats of the subgroup 1A injected with PF before the end of ischemia, diameter of 19 "feeding" arteries at 5 min of reperfusion became smaller. At the same time, in six rats of subgroup 1B (15 arteries) this value differed (P<0.05, Figure 3). During this time, the diameter of mesenteric "feeding" arteries continued to decrease, but differences between groups became less visible after 90 min of reperfusion. In nine rats of subgroup 2A injected with PF before CMA occluding, micro-clamp removing vascular reaction was heterogeneous. The diameter of 15 "feeding" arteries at 5 min of reperfusion was decreased by 10% in comparison with the initial one. In contrast, this value in 10 arteries was increased by 23%. The increase of some feeding artery diameters in post-ischemic period was typically exact for global model of rat's intestinal ischemia.
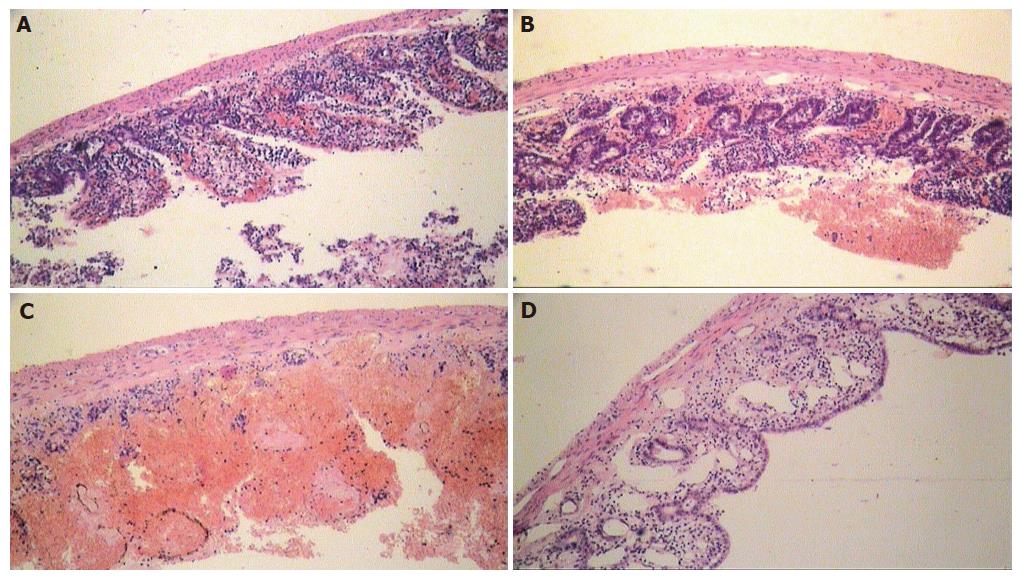
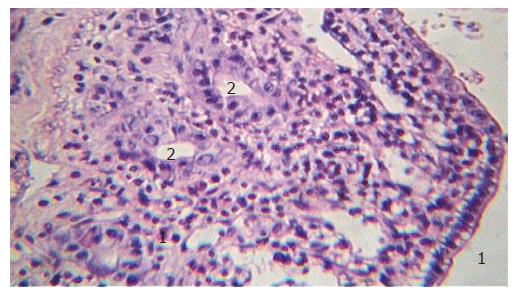
Morphological examination of the intestinal wall sections harvested in group I (partial tourniquet ischemia) only revealed moderate alterations of mucosal layer such as slight apical swelling of intestinal villi with "lifting" of apical epithelium in the ischemic period. But within 90 min after tourniquet removal, degree of intestinal wall alteration progressively increased in comparison to the ischemic period. Therefore, impairment of crypts at 90 min of reperfusion was registered in rats of subgroup IB, spacious parts of intestinal luminal surface were completely denuded with marked villous architecture destruction (Figure 4A). At the same time, in rats of subgroup IA (administered PF) the intestinal wall structural safety was higher in the reperfusion period than in control rats (Figure 4B). Necrobiotic processes and structural changes in rats of subgroup IB progressed with the appearance of focal transmural necroses in the ischemic intestinal wall within 24 h after the end of ischemia (Figure 4C). In rats of subgroup IA (administered PF), alteration was not so significant and even reparative processes such as restoration of villous architecture and epithelial layer regeneration were also marked (Figures 4D and 5). Morphometric analysis data of intestinal wall sections in group I are presented in Table 1.
| Groups | Subgroups | Morphometric indexes | |||
| (number of sections) | Height of number of safety of degree byvilli, μm crypts epithelium (%) Chiu scale | ||||
| Intact | (n = 13) | 497±25 | 143±16 | 100 | 0 |
| Ischemia (90 min) | (n = 8) | 524±16 | 135±44 | 100 | II–III |
| Ischemia (90 min) | IA (n = 5) | 200±11a | 66±16 | 63±18 | III |
| Reperfusion (90 min) | IB (n = 5) | 152±20 | 73±30 | 56±27 | IV |
| Ischemia (90 min) | IA (n = 8) | 304±8a | 77±27a | 70±19a | III |
| Reperfusion (24 h) | IB (n = 9) | 168±28 | 27±33 | 17±20 | IV–V |
Morphological examination of intestinal tissue in group II (occlusion of CMA) revealed a number of characteristic peculiarities. As in a partial intestinal ischemia, moderate changes in the mucosa appeared only at the end of the ischemic period. Then, damage of mucosal and other layers of the intestinal wall progressively increased during 90 min of reperfusion. Destruction of superficial mucosal layer, erythrocyte diapedes, massive hemorrhages, and leukocyte infiltration of the intestinal wall and mesentery occurred in subgroups 2A and B. In general, view was more heterogeneous than in the intestine of group I rats. Moreover, a lot of rats died after 90 min of CMA occlusion in early reperfusion period because of intoxication and progressing circulatory failure. Results of intestinal wall section morphometric analysis in group II are presented in Table 2.
| Groups | Subgroups | Morphometric indexes | |||
| (number of sections) | Height of number of safety ofdegree by villi, μm cryptsepithelium (%) Chiu scale | ||||
| Intact | (n = 2) | 525±19 | 125±24 | 100 | 0 |
| Ischemia (90 min) | IIA (n = 3) | 326±28 | 129±12 | 100 | III |
| Ischemia (90 min) | IIB (n = 7) | 312±18 | 104±13 | 90±10 | III |
| Reperfusion (90 min) | IIA (n = 20) | 296±9 | 118±9 | 97±3 | III |
| Ischemia (90 min) | IIB (n = 24) | 285±9 | 107±6 | 91±4 | III |
The problem of post-ischemic or reperfusion impairments of organ blood flow restoration arises acutely after pharmacological and surgical intervention. Therapeutic measures have been concentrated on a critical threshold, minimization of cell metabolism and degree of tissue microcirculation reperfusion injuries[16-20]. If significant success is achieved in ischemic disorders, the problem of reperfusion injury prophylaxis is far from its final solution and requires not only ways of correction but thorough investigation of its mechanisms. Investigation of the natural phenomena and main mechanisms of reperfusion injuries at "critical" terms of partial and global intestinal ischemia in rats was conducted in the current work.
The tourniquet partial intestinal ischemia model was chosen in the present work for more homogenous pathomorphological receipts. This model reproduces such disorders as incarcerated hernia and other types of bowel strangulation in clinical practice. The second model occluding CMA corresponds to global intestinal ischemia in consequence of arteriomesenteric thrombosis or embolism.
After significant and irreversible structural and functional changes occurred, the "critical term" (point-of-no-return) was about 90 min for tourniquet segmentary intestinal ischemia in rats. The distinct tendency to stable systemic BPM level during ischemic period and its abrupt decrease in early reperfusion period were observed. This BPM level falling was naturally more significant in experiments with CMA occlusion.
Anti-ischemic effect of PF was evaluated in intestinal wall sections from rats that received 0.9% sodium chloride solution. Morphometric analysis was used for quantitative evaluation of rats' intestinal wall alteration degree. A number of morphometric indexes such as the average villous height, number of crypts, epithelial layer safety, and degree of mucosal alteration were analyzed as previously described[15].
The destructive activity increased significantly during reperfusion of ischemic intestine. Mucosa was the most sensitive layer of the intestinal wall. Maximal degree in rat's ischemic intestine was noted at 1.5 h of reperfusion. At the same time, the reperfusion injury strength was significantly smaller in rats that received PF than in controls. In subgroup IA (PF), an explicit regenerative tendency was noted, while destructive process progressed in control rats. In general, luminal surface was covered by low non-mature cubic epithelium, suggesting that crypt's safety is a definitive factor in the viability of ischemic intestine.
It was reported that PFCE has multifunctional properties and is effective in preventing ischemic and reperfusion disorders[21-23]. At the same time, PFCE administration is extremely limited and its mechanisms are poorly understood.
Differences in the intestinal morphologic structure of rats administered PF and control showed the protective role of PF in the reperfusion period. Though no significant destructive changes were found in mesenteric micro-vascular bed, intestinal circulation was restored during reperfusion period. Nevertheless, blood flow restoration during reperfusion was not complete because of “feeding” artery diameter and systemic BPM were significantly smaller than before. At 5 min of reperfusion, feeding artery diameter was decreased by 45% in average compared to the control rats. At the same time, feeding artery diameter was decreased by 24% in rats after PF administration, while BPM level was decreased in both groups (42%), suggesting that there are more feeding arteries after the “critical” ischemia in rats administered PF.
In the analysis of mechanisms of PF protective action on intestinal mucosa, polyfunctional properties of PFCE were taken into account. Therefore, some of them were protected from destruction and mucosal layer regeneration was maintained. Rheological and nitric oxide (NO) transporting properties of PF may take part in this phenomenon.
It is well known that powerful vasodilator NO can protect rat intestine against reperfusion injury[24]. Results of another investigation witness PFCE penetration from blood into endothelial cells of micro-vessels[25], suggesting that PFCE interacts directly with NO in tissues. PF in intestinal microvascular bed may play a role as a NO depot-regulator and protect intestinal wall tissues reperfusion against injury.
PF and NO interactions can be applied to vascular bed of the ischemic intestine. PF may support vasodilatatory potential of mesenteric feeding arteries as a NO depot-regulator during intestinal reperfusion.
In the present study, ischemia-reperfusion systemic and local pathological functional and structural changes were reproduced on experimental models of tourniquet segmentary intestinal loop strangulation and CMA occlusion in rats. "Critical" term (point-of-no-return) for normal thermic tourniquet segmentary intestinal ischemia in rats was about 90 min. Reperfusion could disregulate the systemic blood pressure support system and lead to its abrupt progressive decrease in the early reperfusion period.
In conclusion, the main pathophysiological reperfusion injury of rat’s intestine is the significant mucosal alteration. The degree of intestinal changes is proportional to the duration of ischemia. Systemic administration of perftoran before the reperfusion promotes earlier and more complete structural regeneration in rats with critical partial intestinal ischemia.
Co-first-authors: Vyacheslav Leontjevich Kozhura and Dmitriy Alexeevich Basarab
Co-correspondent: Vasiliy Ivanovich Reshetnyak
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Parks DA, Grøgaard B, Granger DN. Comparison of partial and complete arterial occlusion models for studying intestinal ischemia. Surgery. 1982;92:896-901. [PubMed] |
| 2. | Granger DN. Ischemia-reperfusion: mechanisms of microvascular dysfunction and the influence of risk factors for cardiovascular disease. Microcirculation. 1999;6:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Betzler M. Surgical technical guidelines in intestinal ischemia. Chirurg. 1998;69:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Bjorck M. Colonic ischemia after aortoiliac surgery. Regionar Krovoobr i Microcirc. 2002;1:10-16. |
| 5. | Grant D. Intestinal transplantation: 1997 report of the international registry. Intestinal Transplant Registry. Transplantation. 1999;67:1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 241] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Bauer R, Kerschbaumer F. The results of Chiari's medial displacement osteotomy. Arch Orthop Unfallchir. 1975;81:301-314. [PubMed] |
| 7. | O'Donnell KA, Caty MG, Zheng S, Rossman JE, Azizkhan RG. Oxygenated intraluminal perfluorocarbon protects intestinal muscosa from ischemia/reperfusion injury. J Pediatr Surg. 1997;32:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Kligunenko EN, Kompaniets NG. Possibilities of Perftoran application in complex anti-paretic therapy after operations through laparotomy// Perfluorocarbons in Biology and Medicine: Proc. of Symp./Ed. by G.R. Ivanitsky, V.V.Moroz. Puschino. 2001;133-136, Russian. |
| 9. | Maltseva LA, Mosentsev NF. Effects of Perftoran in patients with septic shock; block of cytokine chain as way of SIRS progression prophylaxis// Perfluorocarbons in Biology and Medicine: Proc. of Symp./Ed. by G.R. Ivanitsky, V.V.Moroz. Puschino. 2001;210-217, Russian. |
| 10. | Ohara M, Unno N, Mitsuoka H, Kaneko H, Nakamura S. Peritoneal lavage with oxygenated perfluorochemical preserves intestinal mucosal barrier function after ischemia-reperfusion and ameliorates lung injury. Crit Care Med. 2001;29:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Vejchapipat P, Proctor E, Ramsay A, Petros A, Gadian DG, Spitz L, Pierro A. Intestinal energy metabolism after ischemia-reperfusion: Effects of moderate hypothermia and perfluorocarbons. J Pediatr Surg. 2002;37:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Brown MF, Ross AJ, Dasher J, Turley DL, Ziegler MM, O'Neill JA. The role of leukocytes in mediating mucosal injury of intestinal ischemia/reperfusion. J Pediatr Surg. 1990;25:214-216; discussion 214-216;. [PubMed] |
| 13. | Bagnenko SF, Korolkov SF, Dzhusoev IG. Effect of Perftoran on regeneration of intestinal anastomoses carried out during hemorrhagic shock experimental investigation// Perfluorocarbons in Medicine and Biology: Proc. of Symp./Ed. by G.R. Ivanitsky, E.B. Zhiburt, E.I. Maevsky. Puschino. 2003;151-154, Russian. |
| 14. | Aleksandrov PN, Chernukh AM. Use of the method of image splitting for vital recording of the diameter of microvessels. Patol Fiziol Eksp Ter. 1972;16:83-85. [PubMed] |
| 15. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1424] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 16. | Bilenko MV. Ischemic and reperfusion injures of organs molecular mechanisms, ways of preventing and treatment. Мoscow: Medicine; 1989. 368 p, Russian. . |
| 17. | Blaisdell FW. The reperfusion syndrome. Microcirc Endothelium Lymphatics. 1989;5:127-141. [PubMed] |
| 19. | Lindsay TF, Luo XP, Lehotay DC, Rubin BB, Anderson M, Walker PM, Romaschin AD. Ruptured abdominal aortic aneurysm, a "two-hit" ischemia/reperfusion injury: evidence from an analysis of oxidative products. J Vasc Surg. 1999;30:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Vlasov TD, Smirnov DA, Nutfullina GM. [Adaptation of the rat small intestine to ischemia]. Ross Fiziol Zh Im I M Sechenova. 2001;87:118-124. [PubMed] |
| 21. | Gennaro M, Mohan C, Ascer E. Perfluorocarbon emulsion prevents eicoasanoid release in skeletal muscle ischemia and reperfusion. Cardiovasc Surg. 1996;4:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Memezawa H, Katayama Y, Shimizu J, Suzuki S, Kashiwagi F, Kamiya T, Terashi A. Effects of fluosol-DA on brain edema, energy metabolites, and tissue oxygen content in acute cerebral ischemia. Adv Neurol. 1990;52:109-118. [PubMed] |
| 23. | Mosca RS, Rohs TJ, Waterford RR, Childs KF, Brunsting LA, Bolling SF. Perfluorocarbon supplementation and postischemic cardiac function. Surgery. 1996;120:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Kubes P. Ischemia-reperfusion in feline small intestine: a role for nitric oxide. Am J Physiol. 1993;264:G143-G149. [PubMed] |
| 25. | Mathy-Hartert M, Krafft MP, Deby C, Deby-Dupont G, Meurisse M, Lamy M, Riess JG. Effects of perfluorocarbon emulsions on cultured human endothelial cells. Artif Cells Blood Substit Immobil Biotechnol. 1997;25:563-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |