Published online Nov 21, 2005. doi: 10.3748/wjg.v11.i43.6863
Revised: April 1, 2005
Accepted: April 2, 2005
Published online: November 21, 2005
AIM: To investigate the incidence and localizations of lymphoid follicles (LFs) in early colorectal neoplasms in human beings.
METHODS: From July 1992 to September 1999, a total of 1 324 early colorectal neoplasms were removed endoscopically or surgically at our hospital; 1 031 (77.9%) were available for analysis in this study. Localization of LFs was defined histologically: as submucosal LFs, if located under the muscularis mucosa; and as intramucosal LFs, if located across or over the muscularis mucosa.
RESULTS: Histologically, the materials included 903 intramucosal neoplasms and 128 submucosal cancers. Overall incidence of LFs was 27.2% (280/1 031). The incidence of LFs was significantly higher in females (33.6% vs 24.9%, P = 0.0064), the right-sided colon (32.2% vs 25.6%, P = 0.0403) and in flat or depressed type lesions (34.6% vs 25.2%, P<0.0001) as compared to males, left-sided colon and protruding type lesions, respectively. The incidences of intramucosal neoplasms and submucosal cancers were 24.3% and 43.8%, respectively (P<0.0001). Localizations of LFs (intramucosal LF/submucosal LF) in depressed, flat, and protruding types were 1/24, 14/36, and 131/74, respectively.
CONCLUSION: The incidence of LFs in early human colorectal neoplasms significantly differs by gender, location, macroscopic type, and histology. Moreover, localization significantly differs by macroscopic type.
- Citation: Fu KI, Sano Y, Kato S, Fujii T, Koba I, Yoshino T, Ochiai A, Yoshida S, Fujimori T. Incidence and localization of lymphoid follicles in early colorectal neoplasms. World J Gastroenterol 2005; 11(43): 6863-6866
- URL: https://www.wjgnet.com/1007-9327/full/v11/i43/6863.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i43.6863
Several reports have investigated the association between lymphoid aggregates and colonic tumors in rodents[1-4]. The results indicate that colonic crypts overlying lymphoid follicles (LFs) show a significantly higher proliferative activity. These results also showed that the risk of carcinoma increased in the colonic mucosa on LFs compared to mucosa without LFs. Consequently, it was considered that factors from LFs promote carcinogenesis in the epithelium in rodents. Rubio et al[5] also investigated the incidence of LFs in early stage adenomas and adenocarcinomas in Swedish and Japanese patients, showing that 38% of 174 consecutive non-polypoid adenomas and flat incipient adenocarcinomas had subjacent LFs. Moreover, they observed a higher incidence of LFs in non-polypoid neoplasms as compared to polypoid neoplasms in rats[6].
Recently, advances in endoscopic techniques and equipment have enabled much smaller flat and depressed colorectal neoplasms to be detected[7-11]. We have also microscopically encountered LFs and found that their incidence and localization tended to be different according to their macroscopic features in early colorectal neoplasms. In this study, we, therefore, aimed to investigate the incidence and localization of LFs revealed in early human colorectal neoplasms.
From July 1992 to September 1999, a total of 1 324 early colorectal neoplasms, including adenoma with high-grade atypia, intramucosal cancer and submucosal invasive cancer, were removed endoscopically or surgically in our hospital. Of the 1 324 lesions, 293 (22.1%) were excluded from this study because the histology had been damaged by endoscopic procedures and they were judged inappropriate for analysis. The lesions of patients with familial polyposis, hereditary non-polyposis colorectal cancer, and inflammatory bowel disease were also excluded.
The locations of the lesions were categorized into two groups at the splenic flexure: right-sided colon (including the cecum, ascending colon, and transverse colon); and left-sided colon (including descending colon, sigmoid colon, and rectum).
Macroscopically, colorectal lesions were classified into three groups according to the criteria of the Japanese Research Society for Cancer of the Colon and Rectum (JRSCCR): depressed (D) type; flat (F) type; and protruding (P) type.
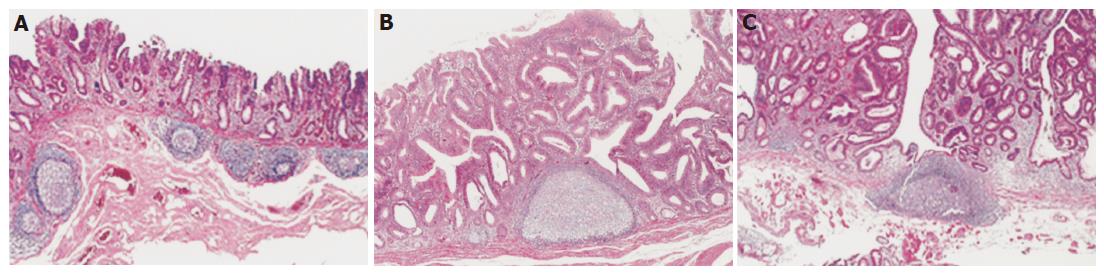
Histological examinations of the available lesions were performed with hematoxylin-and-eosin (HE) staining. The pathological definitions of the lesions were as established by the JRSCCR. We defined a lymphoglandular complex with an enlarged germinal center as a LF. The localizations of LFs were classified into two groups: LFs located beyond the muscularis mucosa were defined as “submucosal LFs” (Figure 1A); and LFs located across or above the muscularis mucosa were defined as “intramucosal LFs” (Figures 1B and C). The presence and localization of LFs were investigated histologically.
Using χ2 test and Fisher′s exact probability test, we compared the clinicopathological characteristics and the incidence and localization of LFs in early human colorectal neoplasms. Correlations between lesion size and the presence or absence of LFs in each macroscopic type were evaluated by using Spearman’s rank correlation. A value of P<0.05 was considered statistically significant.
The details of the clinicopathological characteristics of the subjects are shown in Table 1. A total of 1 031 lesions, including 903 intramucosal neoplasms and 128 submucosal cancers, were investigated histologically. Endoscopically, 60 were depressed type, 157 flat type, and 814 protruding type.
| LF1 present (%) | LF absent (%) | P | ||
| Total cases | 1 031 | 280 (27.2) | 51 (72.8) | |
| Gender | ||||
| Male | 766 | 191 (24.9) | 575 (75.1) | |
| Female | 265 | 89 (33.6) | 176 (66.4) | 0.0064 |
| Location | ||||
| Right - sided colon | 245 | 79 (32.2) | 166 (67.8) | |
| Left - sided colon | 786 | 201 (25.6) | 585 (74.4) | 0.0403 |
| Size (mean)(mm) | ||||
| Depressed + Flat | 10.3 ± 3.4 | 13.9 ± 6.3 | 8.5 ± 5.3 | |
| Protruding | 14.1 ± 9.6 | 17.9 ± 12.0 | 12.8 ± 8.3 | 0.0095 |
| Macroscopic type | ||||
| Depressed + Flat | 217 | 75 (34.6) | 142 (65.4) | |
| Protruding | 814 | 205 (25.2) | 609 (74.8) | 0.0133 |
| Histology | ||||
| Intramucosal neoplasias | 903 | 224 (24.8) | 679 (75.2) | |
| Submucosal cancer | 128 | 56 (43.8) | 72 (56.2) | <0.0001 |
LFs were present in 280 of the 1 031 lesions, an overall incidence of 27.2%. The incidence of LFs was significantly higher in females (89/265, 33.6%) compared to males (191/766, 24.9%) (P = 0.0064). The incidence of LFs was markedly higher in the right-sided colon (32.2%, 79/166) than in the left-sided colon (25.6%, 201/786, P = 0.0403). In addition, the incidence of LFs in submucosal cancers (43.8%, 56/128) was significantly higher than in intramucosal neoplasms (24.3%, 224/903, P<0.0001). The incidences of LFs in submucosal cancers with or without lymph node metastasis were 8.9% (5/56) and 8.3% (6/72), respectively, showing no significant correlation between the presence of LFs and lymph node metastasis in submucosal cancers. LFs were present in 41.7% (25/60) of D type, 31.8% (50/157) of F type, and 25.2% (205/814) of P type lesions. The incidence of LFs in F and D type (34.6%, 75/217) was significantly higher than in P type (25.2%, 205/814) (P<0.0001, Table 1).
Of the 280 lesions with LFs, 146 were classified as intramucosal and 134 as submucosal. In addition, the localizations of LFs (intramucosal/submucosal) were also different according to macroscopic types: 1/24 in D, 14/36 in F, and 131/74 in P types (Table 2). The D and F type lesions harboring LFs also showed a significantly higher incidence of submucosal LFs as compared to the P type lesions (P<0.0001).
The mean tumor sizes of the early colorectal neoplasms with or without LFs in each macroscopic type were 12.3±6.3 and 8.0±5.7 mm in D type, 14.8±10.6 and 8.6±5.1 mm in F type, and 17.9±12.0 and 12.8±8.3 mm in P type, respectively, which showed that the mean tumor size in each macroscopic type with LFs was obviously larger compared to the macroscopic type without LFs (P<0.0001), and the mean tumor sizes of D and F types with LFs were markedly smaller than that of P type (P = 0.0095).
We believe that this study is the first report to describe the incidence and localization of LFs subjacent to early colorectal neoplasms in human beings. Rubio et al[5,6] concluded that there appeared to be a genuine association between LFs and non-polypoid adenomas, not only in rodents but also in human beings; however, they did not mention depressed type lesions. Recently, with advances in endoscopic instruments and techniques, concerns have arisen about depressed and flat type early colorectal neoplasms that have increasingly been found worldwide[7-11]. To clarify the characteristics of these depressed and flat type lesions, we investigated the incidence of LFs not only of protruding, but also of depressed and flat type lesions.
Our present results showed that the incidence of LFs differed according to the macroscopic features of early colorectal neoplasms, being especially high in depressed type lesions (41.7%). We also observed that the localizations of LFs seen in early colorectal neoplasms were different according to macroscopic type: 96% (24/25) of depressed and 72% (36/50) of flat lesions-associated LFs were located under the muscularis mucosa (submucosal LF), while 36% (74/205) of protruding lesions harboring LFs were located over or across the muscularis mucosa (intramucosal LF). To the best of our knowledge, no other reports on the localization of LFs in early colorectal neoplasms have been published as yet.
Depressed lesions are known to demonstrate invasive tendencies despite their smaller size[7-9]. Furthermore, flat lesions are reported to be 10 times more likely to contain high-grade dysplasia than protruding ones[12]. In this study, the depressed or flat lesions were found to have a significantly higher incidence of LFs and submucosal LFs compared to the protruding lesions. Based on reports of the aggressive growth patterns of depressed and flat lesions, we speculate that the differences in incidence and location of LFs between flat or depressed and protruding lesions might reflect the host physical defense against neoplasms in human beings; that the submucosal LFs might prevent depressed or flat lesions from downward growth; and that LFs might act against the upward growth of protruding lesions. However, the results from experimental colon cancer studies indicated that aggregates of LFs might promote the development of adenocarcinomas[1-4]. On the other hand, studies in experimental animals have also shown that the intestinal lymphoid system plays an important role in the immunologic defense mechanisms; that is, antigenic stimuli result in germinal center formation, subsequently antibody production, and finally enlargement of the follicles[13]. In vivo, the presence of tumor-infiltrating lymphocytes is associated with improved prognosis in colorectal cancers, as does the presence of high level DNA microsatellite instability[14-15]. Carcinomas with lymphoid stroma in various organs are also reported to be associated with better prognosis[16]. Thus, these results suggest LFs in early colorectal neoplasms play an important role in defense rather than promotion.
In our study, the incidence of LFs was markedly higher in submucosal invasive cancers than in intramucosal lesions, and it was also more frequently observed in depressed or flat lesions and in larger sizes of each macroscopic type. We observed significant correlations among the incidence of LFs and the invasive tendencies and size of early colorectal lesions. Mortality rates for colorectal cancer in Japan tend to be lower in females than males. Our results also showed a significant higher incidence of LFs in females compared to males (33.6% vs 24.9%). Taking these results together, we suggest that LFs in early colorectal neoplasms are signs of a possible early physical defense event against neoplastic cells.
Submucosal cancers are reported to show lymph node metastasis in 3.6–16.2%[17-20]. However, in this study, we could not find a significant difference between the presence of LFs and lymph node metastasis in submucosal cancers. This might be due to the small numbers of submucosal cancers (8.6%, 11/128) harboring lymph node metastasis. Another explanation is that LFs may have defense only against the process of tumor invasion but not that of metastasis.
In conclusion, significant differences exist in the incidence and localization of LFs in early colorectal neoplasms in human beings. We suggest that LFs in early colorectal neoplasms might be considered as a sign of the host physical defense against neoplastic cells. Further studies, including experimental and clinical analyses, will be necessary to confirm this phenomenon.
Science Editor Kumar K and Guo SY Language Editor Elsevier HK
| 1. | Nauss KM, Locniskar M, Pavlina T, Newberne PM. Morphology and distribution of 1,2-dimethylhydrazine dihydrochloride-induced colon tumors and their relationship to gut-associated lymphoid tissue in the rat. J Natl Cancer Inst. 1984;73:915-924. [PubMed] |
| 2. | Hardman WE, Cameron IL. Colonic crypts located over lymphoid nodules of 1,2-dimethylhydrazine-treated rats are hyperplastic and at high risk of forming adenocarcinomas. Carcinogenesis. 1994;15:2353-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Ward JM. Morphogenesis of chemically induced neoplasms of the colon and small intestine in rats. Lab Invest. 1974;30:505-513. [PubMed] |
| 4. | Martin MS, Hammann A, Matin F. Gut-associated lymphoid tissue and 1,2-dimethylhydrazine intestinal tumors in the rat: a histological immunoenzymatic study. Int J Cancer. 1986;38:75-80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Rubio CA, Kumagai J, Kanamori T, Nakamura K. Apoptosis in flat neoplasias of the colorectal mucosa. In Vivo. 1995;9:173-176. [PubMed] |
| 6. | Rubio CA, Shetye J, Jaramillo E. Non-polypoid adenomas of the colon are associated with subjacent lymphoid nodules. An experimental study in rats. Scand J Gastroenterol. 1999;34:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Kudo S, Tamura S, Hirota S, Sano Y, Yamano H, Serizawa M, Fukuoka T, Mitsuoka H, Nakajima T, Kusaka H. The problem of de novo colorectal carcinoma. Eur J Cancer. 1995;31A:1118-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Kudo S, Tamure S, Nakajima T, Hirota S, Asano M, Ito O, Kusaka H. Depressed type of colorectal cancer. Endoscopy. 1995;27:54-57; discussion 61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Fujii T, Rembacken BJ, Dixon MF, Yoshida S, Axon AT. Flat adenomas in the United Kingdom: are treatable cancers being missed? Endoscopy. 1998;30:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Hart AR, Kudo S, Mackay EH, Mayberry JF, Atkin WS. Flat adenomas exist in asymptomatic people: important implications for colorectal cancer screening programmes. Gut. 1998;43:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, Axon AT. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 410] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 12. | Wolber RA, Owen DA. Flat adenoma of the colon. Hum Pathol. 1991;34:981-986. |
| 13. | O'Leary AD, Sweeney EC. Lymphoglandular complexes of the colon: structure and distribution. Histopathology. 1986;10:267-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 130] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Michael-Robinson JM, Biemer-Huttman A-E, Purdie DM, Walsh MD, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL. Tumor infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360-366. [RCA] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Minamoto T, Mai M, Watanabe K, Ooi A, Kitamura T, Takahashi Y, Ueda H, Ogino T, Nakanishi I. Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer. 1990;66:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Kyzer S, Bégin LR, Gordon PH, Mitmaker B. The care of patients with colorectal polyps that contain invasive adenocarcinoma. Endoscopic polypectomy or colectomy? Cancer. 1992;70:2044-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Minamoto T, Mai M, Ogino T, Sawaguchi K, Ohta T, Fujimoto T, Takahashi Y. Early invasive colorectal carcinomas metastatic to the lymph node with attention to their nonpolypoid development. Am J Gastroenterol. 1993;88:1035-1039. [PubMed] |
| 19. | Tanaka S, Yokota T, Saito D, Okamoto S, Oguro Y, Yoshida S. Clinicopathologic features of early rectal carcinoma and indications for endoscopic treatment. Dis Colon Rectum. 1995;38:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Tanaka S, Haruma K, Teixeira CR, Tatsuta S, Ohtsu N, Hiraga Y, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol. 1995;30:710-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 107] [Article Influence: 3.6] [Reference Citation Analysis (0)] |