Published online Nov 21, 2005. doi: 10.3748/wjg.v11.i43.6848
Revised: April 6, 2005
Accepted: April 9, 2005
Published online: November 21, 2005
AIM: To determine the predictive factors for hepatocellular carcinoma (HCC) development in patients after spontaneous or therapeutic HBeAg seroconversion.
METHODS: In 48 patients who seroconverted to anti-HBe positive during follow-up, the background factors for HCC development were analyzed.
RESULTS: HCC was developed in six patients during follow-up (average follow-up after HBeAg seroconversion: 10.9±5.4 years). The incidence of HCC evaluated by Kaplan–Meier analysis was significantly higher in patients with abnormal aspartate aminotransferase (AST> 40 IU/L) level, lower platelet counts (PLT<10×104/µL), lower albumin level (Alb<30 g/L), positive HBV-DNA or older age at seroconversion (>40 years). However, lower platelet count was the only predictive factor for HCC development shown by multivariate proportional-hazard analysis.
CONCLUSION: Active hepatitis or advanced hepatitis at HBeAg seroconversion or progressive hepatitis even after HBeAg seroconversion would be the risk factors for HCC development. These predictive factors should be taken into account in determining the frequency of biochemical study or imaging studies for HCC surveillance.
- Citation: Murata K, Sugimoto K, Shiraki K, Nakano T. Relative predictive factors for hepatocellular carcinoma after HBeAg seroconversion in HBV infection. World J Gastroenterol 2005; 11(43): 6848-6852
- URL: https://www.wjgnet.com/1007-9327/full/v11/i43/6848.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i43.6848
Hepatitis B virus (HBV) in Japan, where genotype C is dominant, is generally infected during delivery or early childhood with immature immune systems, and those children become HBV carriers before HBV vaccine was introduced. In adolescents, hepatitis e antigen (HBeAg) seroconversion to its antibody (anti-HBe) often occurs after multiple exacerbations, which coincide with subsidence of hepatic inflammatory activities[1,2]. The failure of seroconversion usually results in chronic progressive liver diseases and hepatocellular carcinoma (HCC). The calculated annual incidence of acute exacerbation was two times higher in patients with positive HBeAg or HBV-DNA than those without these markers[3]. The patients who have recurrent episodes of acute exacerbations with bridging hepatic necrosis are more likely to develop cirrhosis[4]. These observations suggest that HBeAg seroconversion to anti-HBe confers favorable long-term outcomes. On the other hand, most HCC occurred in HBeAg-negative patients with undetectable HBV-DNA[5,6], suggesting that all HBV patients should be carefully followed-up for HCC even after HBeAg seroconversion. However, there seems to be some predictive factors for HCC in our clinical experiences. Previous reports demonstrated the natural history of anti-HBe positive patients. However, they included patients with spontaneous reactivation, which may be the major cause of progressive hepatic damage[7,8]. To our knowledge, there are no reports about incidences of HCC that selected only patients who seroconverted to anti-HBe during follow-up. Therefore, we focused on anti-HBe-positive patients without any reactivation of HBeAg and tried to determine the risk factors for HCC development to select high risk group for HCC.
Three hundred and thirty-one HBsAg-positive patients were followed-up in our hospital between 1990 and 2004. Inclusion criteria in this study were: (1) follow-up until death or October 2004; (2) sufficient data to evaluate; and (3) careful follow-up for HCC with evaluation of both tumor markers (alpha-fetoprotein or des-gamma-carboxyprothrombin) and image studies (USG or CT) every 3-6 mo. HBeAg serocoversion was determined if anti-HBe completely became positive and HBeAg was never recovered retrospectively. We excluded the cases that showed fluctuation of HBeAg or anti-HBe levels. None of the patients had any other viral infections, such as hepatitis C virus (HCV) or human immunodeficiency virus (HIV). Patients were followed-up by biochemical studies and image studies for at least 3-6 mo intervals. The biochemical tests were measured using routine automated techniques. HBsAg, HBeAg, and anti-HBe were assayed using commercial enzyme immunoassay kits (Abbott Japan, Tokyo). HBV-DNA was determined by a transcription-mediated amplification (TMA) method. The detection sensitivity of this assay is 3.7 LGE/mL. All HCC were initially found by USG or CT and confirmed by CT during abdominal angiography.
Fisher’s exact test or the χ2 test was used to compare patients’ backgrounds. Kaplan–Meier analysis and log-rank test were performed to test a cumulative incidence of HCC in each group. Multivariate proportional-hazards survival analysis was used to determine the important risk factors for HCC. P<0.05 was considered statistically significant.
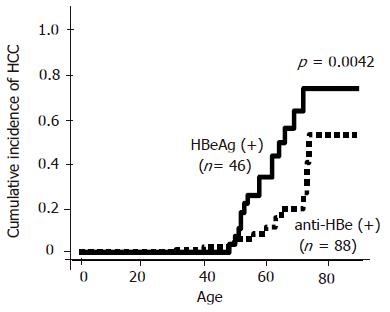
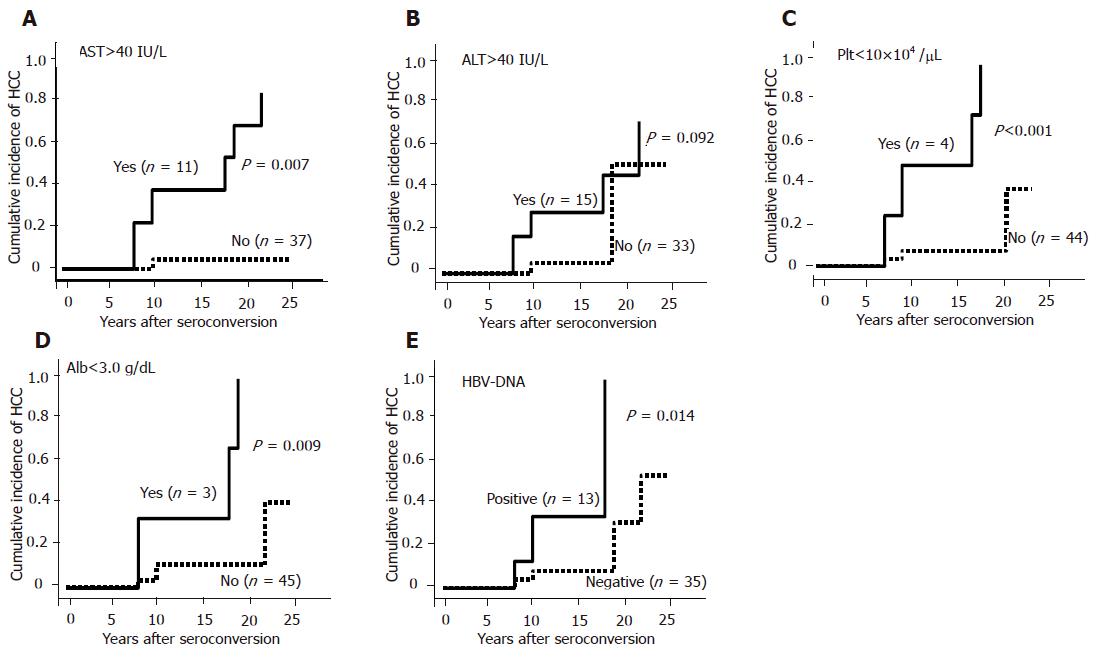
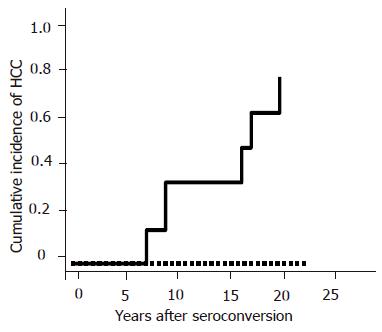
One hundred and thirty-four out of the 331 HBV patients were enrolled in this study. Forty six patients (M:F = 34:12) were HBeAg-positive and 88 (M:F = 64:24) patients were anti-HBe-positive. There were no patients who had positive tests for both HBeAg and anti-HBe. Regarding the patients’ backgrounds, age, sex, platelet counts, albumin level and AST level were similar in both HBeAg-positive and anti-HBe-positive patients. However, ALT level was significantly higher in HBeAg-positive patients as compared with anti-HBe-positive patients (P = 0.001, Table 1 ). HCC developed in 15 HBeAg-positive patients and in 11 anti-HBe-positive patients during follow-up. The average age for the appearance of HCC was similar in both groups (55±8 years in HBeAg-positive and 53±12 years in anti-HBe-positive patients). The cumulative incidence of HCC was significantly higher in HBeAg-positive patients than in anti-HBe-positive patients as previously reported (P = 0.016, Figure 1). However, the incidence of HCC was found to be increased with age even in patients with anti-HBe, especially in those who were over 70 years. Out of 88 patients with anti-HBe, the time of HBeAg seroconversion was observed in 48 patients. Other 40 patients already showed anti-HBe positive when the patients first presented. The current study focused on these 48 patients. The average age of 48 patients was 50±14 years (M:F = 34:14). The average age at HBeAg seroconversion was 40±12 years (range, 16-67 years). HBeAg seroconversion occurred spontaneously in 75% (36/48), by IFN in 18.8% (9/48) and by propagermanium in 6.2% (3/48) cases. HBV-DNA was detectable by TMA methods in 27.1% (13/48) patients. The average follow-up periods after HBeAg seroconversion were 10.9±5.4 years (range, 1-25 years). Six patients developed HCC during follow-up. All six patients were male and the average age of HCC development was 57±13 years (14.0±5.1 years after HBeAg seroconversion). The incidence of HCC evaluated by Kaplan–Meier analysis was significantly higher in patients with abnormal AST (>40 IU/L) level (P = 0.007, Figure 2A), suggesting that active hepatitis even after seroconversion may be one of the risk factors for HCC. However, there were no differences in ALT levels (Figure 2B). Low platelet count (<10×104/µL) (P<0.0001) or low albumin level (Alb<30 g/L) (P = 0.009) was another risk factor for HCC, suggesting that HCC may develop in patients with advanced liver diseases at HBeAg seroconversion or with progressive liver diseases even after seroconversion (Figures 2C and D). Positive HBV-DNA (P = 0.014) increased the incidence of HCC as well (Figure 2E). The incidence of HCC tended to be non-significantly higher in the patients with HBeAg seroconversion by therapy as compared with the spontaneous seroconversion (Figure 3A). The cumulative incidence of HCC was significantly higher in the patients with HBeAg seroconversion after 40 years of age (Figure 3B). Multivariate proportional-hazards analysis demonstrated that low platelet counts (<10×104/µL) was the only predictive factor for HCC (P = 0.032, hazard ratio = 21.6), whereas serum albumin and AST levels and HBV-DNA positivity could not be determined as predictive factors for HCC (Table 2). Furthermore, the incidence of HCC was not observed in the patients with both normal platelet counts (>10×104/µL) and normal AST level (<40 IU/L) during follow-up in our study. In contrast, the incidence of HCC was annually increasing after HBeAg seroconversion in patients with either lower platelet counts (<10×104/µL) or abnormal AST level (>40 IU/L) (Figure 4).
| Feature | HbeAg | Anti-Hbe | P |
| n | 46 | 88 | NS |
| Age | 53±12 | 54±14 | NS |
| Male sex | 34 (74%) | 64 (73%) | NS |
| Platelet (×104/µL) | 14.3±6.6 | 18.7±6.4 | NS |
| Alb (g/dL) | 4.0±0.5 | 4.1±0.5 | NS |
| AST (IU/L) | 56±33 | 41±36 | NS |
| ALT (IU/L) | 62±57 | 36±27 | 0.001 |
| Variable | Hazard ratio | 95%CI | P |
| Platelet counts | 21.6 | 1.3 – 354.8 | 0.032 |
| (< 105vs≥ 105/mL) | |||
| Alb (< 3.0 vs≥ 3.0 g/dL) | 0.4 | 0.1 – 3.4 | 0.376 |
| AST (> 40 vs ≤ 40 IU/L) | 3.6 | 0.2 – 51.3 | 0.351 |
| HBV-DNA (+ vs -) | 0.1 | 0.1 – 10.4 | 0.912 |
The present study demonstrated that low platelet counts (<10×104/µL), low albumin level (<30 g/L), abnormal AST level (>40 IU/L), older age at HBeAg seroconversion (>40 years) and positive HBV-DNA were the predictive factors for HCC after HBeAg seroconversion. Multivariate proportional-hazard analysis demonstrated that platelet count was the only significant risk factor. These observations suggested that active hepatitis or progression of liver disease even after HBeAg seroconversion or advanced hepatitis at HBeAg seroconversion would be the predictive factors for HCC. In other words, patients without any of these factors after HBeAg seroconversion would be at low risk for HCC development.
In patients with chronic hepatitis C virus (HCV) infection, we should focus on patients with advanced chronic hepatitis or cirrhosis as high risk group for HCC development and follow-up with tight observation for biochemical studies, tumor markers and image studies since HCC is usually accompanied with the progression of liver diseases. In patients with chronic HBV infection, however, HCC sometimes develops not only in liver cirrhosis, but even in mild hepatitis[9,7]. So, it is more difficult to select high risk patients for HCC in chronic HBV infection than that in HCV infection. Of course, positive HBeAg or reactivation after HBeAg seroconversion with active hepatitis is one of the risk factors for cirrhosis[7,8], HCC[3,10] or survival[11]. However, the cumulative incidence of HCC in our study was high even in anti-HBe-positive patients, especially in older patients (Figure 1). Therefore, we conducted this study to determine the predictive factors for HCC in patients with anti-HBe.
We found that the cumulative incidence of HCC was significantly higher in patients with abnormal AST (Figure 2A), suggesting that active hepatitis even after HBeAg seroconversion was one of the risk factors. This result was consistent with previous reports that progression to cirrhosis or HCC occurred in patients with HBeAg negative hepatitis without reversion to HBeAg[10,12]. Furthermore, the incidence of HCC was significantly reduced in HBeAg-negative chronic hepatitis or cirrhotic patients who maintained virological response by lamivudine[13] or interferon (IFN)[14,15]. These previous reports support our observation that therapy for transaminase control may reduce the risk of HCC development in our patients with abnormal AST level. On the other hand, the cumulative incidence of HCC was not statistically different in ALT levels. We, therefore, hypothesized that ALT elevation might be observed in patients with fatty liver as well as necroinflammatory liver disease.
Other risk factors for HCC development in anti-HBe-positive patients were lower platelet counts and low albumin level. Interestingly, multivariate proportional-hazards analysis showed that low platelet counts (<10×104/µL) was the only risk factor for HCC development, suggesting that HCC may develop in patients with progressive liver disease even after HBeAg seroconversion or advanced liver diseases before HBeAg seroconversion. Since multiple exacerbations usually occurred before HBeAg seroconversion, necroinflammation occurs more frequently in patients with delayed HBeAg seroconversion, which results in progression to liver cirrhosis or HCC[12]. Our results show that the cumulative incidence of HCC was significantly higher in patients with delayed HBeAg seroconversion (older than 40 years) are consistent with the previous reports. Furthermore, the cumulative incidence of HCC in our study was annually increasing in the patients with either low platelet counts (<10×104/µL) or abnormal AST (>40 IU/L) after HBeAg seroconversion, whereas no HCC was observed in patients with both normal platelet counts (≥10×104/µL) and normal AST (<40 IU/L) during follow-up (median follow-up period: 11±5 years).
In this study, we observed a relatively higher incidence of HCC in patients with anti-HBe as compared with the previous reports. This might be because our study was not a prospective study and we might have lost the patients with relative low risk of active hepatitis or fibrosis during follow-up. It is possible that there might be a bias, in which the patients with relative high risk (high AST or ALT) were accumulated in our study. Prospective and large-scale studies need to determine the predictive factors for HCC in patients with anti-HBe.
In conclusion, in anti-HBe-positive patients, lower platelet counts, lower albumin, abnormal AST and positive HBV-DNA are the predictive factors for HCC. In other words, anti-HBe-positive patients without any of these factors are at low risk for HCC. In the follow-up of anti-HBe-positive patients, those predictive factors, especially platelet counts, should be taken into account for determining the frequency of biochemical study or imaging studies. Moreover, to reduce the risk for HCC development, we should induce HBeAg seroconversion before progression to advanced liver diseases and control AST level within the normal range using lamivudine or IFN. These results may be supported by our clinical experiences. However, to our knowledge, there are no reports to clarify the high risk group in anti-HBe positive patients with known time of HBeAg seroconversion.
Science Editor Kumar M and Guo SY Language Editor Elsevier HK
| 1. | Hoofnagle JH, Dusheiko GM, Seeff LB, Jones EA, Waggoner JG, Bales ZB. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann Intern Med. 1981;94:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 432] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Realdi G, Alberti A, Rugge M, Bortolotti F, Rigoli AM, Tremolada F, Ruol A. Seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B virus infection. Gastroenterology. 1980;79:195-199. [PubMed] |
| 3. | Liaw YF, Lin DY, Chen TJ, Chu CM. Natural course after the development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Liver. 1989;9:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Chu CM. Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2000;15 Suppl:E25-E30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303-S309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Yuen MF, Lai CL. Natural history of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2000;15 Suppl:E20-E24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Fattovich G, Brollo L, Alberti A, Realdi G, Pontisso P, Giustina G, Ruol A. Spontaneous reactivation of hepatitis B virus infection in patients with chronic type B hepatitis. Liver. 1990;10:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 244] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Di Bisceglie AM, Rustgi VK, Hoofnagle JH, Dusheiko GM, Lotze MT. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988;108:390-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 321] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 11. | Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, Häussinger D. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med. 1996;334:1422-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 574] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Marco VD, Marzano A, Lampertico P, Andreone P, Santantonio T, Almasio PL, Rizzetto M, Craxi A for the Italian Association for the Study of the Liver (AISF) Lamivudine Study Group. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40:883-891. [RCA] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Di Marco V, Lo Iacono O, Cammà C, Vaccaro A, Giunta M, Martorana G, Fuschi P, Almasio PL, Craxì A. The long-term course of chronic hepatitis B. Hepatology. 1999;30:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Papatheodoridis GV, Manesis E, Hadziyannis SJ. The long-term outcome of interferon-alpha treated and untreated patients with HBeAg-negative chronic hepatitis B. J Hepatol. 2001;34:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |