Published online Nov 21, 2005. doi: 10.3748/wjg.v11.i43.6828
Revised: June 6, 2005
Accepted: June 9, 2005
Published online: November 21, 2005
AIM: To evaluate a series of patients with hepatocellular carcinoma (HCC) treated with several different protocols and devices.
METHODS: We treated 138 patients [chronic hepatitis/liver cirrhosis (Child–Pugh A/B/C), 3/135 (107/25/3)] with two different devices and protocols: cool-tip needle [initial ablation at 60 W (standard method) (n = 37) or at 40 W (modified method) (n = 28)] or; ablation with a LeVeen needle using a standard single-step, full expansion (single-step) method (n = 39) or a multi-step, incremental expansion (multi-step) method.
RESULTS: Eleven patients experienced rapid and scattered recurrences 1 to 7 mo after the ablation. Nine patients were treated by the cool-tip original protocol (60 W) (9/37 = 24%) and the other two by the LeVeen single-step method (2/39 = 5%). The location of the recurrence was surrounding and limited to the site of ablation segment in three cases, and spread over one lobule or both lobules in the other eight cases. There was no recurrence in the patients treated with the modified cool-tip modified method (40 W) or the LeVeen multi-step method.
CONCLUSION: There is a risk of rapid and scattered recurrence after RFA, especially when the standard cool-tip procedure is used. Because such recurrence would worsen the prognosis, we recommend that modified protocols for the cool-tip and LeVeen needle methods should be used in clinical practice.
- Citation: Kotoh K, Enjoji M, Arimura E, Morizono S, Kohjima M, Sakai H, Nakamuta M. Scattered and rapid intrahepatic recurrences after radio frequency ablation for hepatocellular carcinoma. World J Gastroenterol 2005; 11(43): 6828-6832
- URL: https://www.wjgnet.com/1007-9327/full/v11/i43/6828.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i43.6828
Image-guided radio frequency ablation (RFA) is an emerging technique for the treatment of hepatocellular carcinoma (HCC)[1-5], as well as for metastatic liver tumors[6]. The procedure has been adopted worldwide as a safe and effective method, and is replacing percutaneous ethanol injection therapy (PEIT) as the treatment of choice. Livraghi et al[7] and Lencioni et al[8] have compared RFA and PEIT for the treatment of small-sized HCC. Although both studies concluded that RFA resulted in a higher rate of complete necrosis, Livraghi et al[7] also indicated that the complication rate was higher with RFA than with PEIT.
A number of reports have described complications associated with RFA[9-14]. The major complications reported were peritoneal bleeding, hepatic abscess, hemothorax, perforation of the gastrointestinal wall, and rapid hepatic decompensation. These complications occurred during or just after RFA, however, and delayed complications have been reported much less frequently. Takada et al[15] described two cases in which rapid and aggressive recurrence accompanied by portal thrombus occurred 4 to 6 mo after RFA. Nicoli et al[16] described a peculiar form of recurrence after RFA for HCC that was characterized by numerous and equally-sized recurrence nodules and which occurred after only one month post-treatment. More recently, Ruzzenente et al[17] also described a series of patients with rapidly spreading recurrence after RFA.
In the past several years, we have observed cases similar to those presented by Nicoli et al[16]. We have previously reported a significant increase in pressure in the ablated area during RFA[18], and concluded that scattered recurrence is attributable to an explosion caused by excessive increases in intra-tumor pressure. In our experience, intra-operative complications are easily avoidable by confirming the safety of a puncture route before the treatment, whereas a rapid recurrence after RFA is a serious clinical problem that can influence the prognosis of patients.
In this report, we have evaluated a series of HCC cases treated by RFA with different devices and protocols, and have analyzed cases with rapid and scattered recurrence after RFA.
Between April 2000 and December 2004, 138 patients with HCC were treated with the RFA procedure in Kyushu University Hospital or National Hospital Organization Kyushu Medical Center. Characteristics of the candidates are shown in Table 1. They consisted of 79 males and 59 females, aged 40 to 83 years with a mean of 68.2 years. All of them had chronic liver damage; 121 patients were hepatitis C virus (HCV) positive and 17 were hepatitis B virus (HBV) positive. Among them, 135 patients were diagnosed with liver cirrhosis by liver biopsy, clinical laboratory data, ultrasonography and/or computed tomography. According to the hepatic functional reserve evaluation for the cirrhotic patients just before RFA, 107, 25, and 3 were classified as Child–Pugh’s class A, B, and C, respectively. The diagnosis of HCC was confirmed by aspiration tumor biopsy for all patients prior to treatment.
| Scattered recurrence (–) | Scattered recurrence (+) | Total | |
| n | 127 | 11 | 138 |
| Age | 67.5±8.6 | 69.2±8.1 | 67.6±8.5 |
| Sex (male/female | 71/56 | 8月3日 | 79/59 |
| Virus (HBV/HCV) | 14/113 | 3月8日 | 17/121 |
| Tumor size (mm) | 24.3±13.7 | 20.1±5.7 | 24.0±13.5 |
| Albumin (g/dL) | 3.65±0.38 | 3.52±0.57 | 3.64±0.39 |
| Bilirubin (mg/dL) | 0.86±0.51 | 1.02±0.44 | 0.87±0.50 |
| ALT (U/L) | 48.4±28.3 | 56.0±17.8 | 49.0±28.5 |
| Platelet (x104/mL) | 10.9±5.5 | 9.0±3.6 | 10.7±5.5 |
| Child–Pugh (CH1/A/B/C)a | 3/102/20/2 | 0/5/5/1 | 3/107/25/3 |
| Device and Protocolb | |||
| Original cool-tip | 28 | 9 | 37 |
| Modified cool-tip | 28 | 0 | 28 |
| Single-step LeVeen | 37 | 2 | 39 |
| Multi-step LeVeen | 34 | 0 | 34 |
| Prognosis (alive/death)b | 87/40 | 3月8日 | 90/48 |
| Observation period (mo) | 27.1±11.0 | 24.0±11.5 | 26.9±11.1 |
RFA was performed with either a LeVeen™ multipolar array needle in combination with an RF 2000 generator™ (Radio Therapeutics Corporation, Mountain View, CA, USA) or a Cool-tip™ RF System (3.0 cm exposure length) (Radionics, Burlington, MA, USA). One of the two devices was selected randomly for RFA. All procedures were performed by hepatologists who had at least 10 years of experience performing image-guided in situ tumor ablation therapy. The original standard protocol was used for cool-tip needle RFA until December 2002, and the modified protocol was used thereafter. For LeVeen needle RFA, the original standard protocol was used until April 2002, and the modified method was used thereafter. The details of each protocol are described below.
Original procedure with cool-tip needle: Cool-tip electrode with 3 cm of exposed tip was used to deliver RF (radio frequency) energy to the tumors. RF energy was delivered as described previously[19]. In short, after needle puncture of the tumor, generator output was increased to 100–120 W and maintained at this level until the end of the procedure. If an increase in impedance equal to or greater than 10 Ω above baseline was observed, the current was reduced, until stable impedance was observed and then increased again.
Modified procedure with cool-tip needle: The needle used was the same type as was used for the original procedure. The method differed from the original procedure in that the ablation was started at a low voltage of 40 W, and the electric power was increased by 10 W every minute. The maximum of electric power was 120 W, and the RF energy delivery was continued, until the impedance increased beyond the limit of the generator.
Original procedure with LeVeen needle: The electrode used for this procedure was a 3 or 3.5 cm LeVeen needle depending on the tumor size. Before delivery of RF power, the tumor was punctured with a needle and the ten tines were then fully expanded. The ablation was started at 40 W (3-cm needle) or 50 W (3.5-cm needle) RF power and was further increased by 10 W/min up to 75 W (3-cm needle) or 90 W (3.5-cm needle). If the impedance had not increased after 10 min, the RF power was again increased by 10 W increments. The procedure was terminated when a marked increase in impedance (“roll off”) occurred.
Modified procedure with LeVeen needle: The needle used was the same type as was used for the original procedure. In the modified version, the tines of the electrode were expanded step by step in 10 steps, and at every step, the length of tine expansion was one-tenth of the full expansion length. Ablation at each step was continued, until the impedance increased to “roll off”. Furthermore, at the first step, the ablation was started at a low voltage of 30 W. If it took more than 30 s for “roll off” at a step, the power was increased by 10 W before starting the next step. The maximum electric power for this protocol was 75 W (3-cm needle) or 90 W (3.5-cm needle), which was maintained until the final step.
After RFA treatment, all of the patients were followed up every one or two months with US or CT. When recurrence was detected by imaging examination, additional treatment was instituted with RFA, PEIT, transarterial chemoembolization (TACE) or a combination of these therapies. The prognosis was based on the data obtained up to December 2004.
Baseline characteristics of the patients prior to RFA treatment are shown as mean±SD and statistical comparisons were performed using χ2 test for categorical data and non-paired t-test for numerical data.
RESULTS
Eleven patients suffered from rapid and scattered intrahepatic recurrences, which occurred between one month and seven months after ablation. These 11 cases consisted of eight males and three females, ranging in age from 51 to 79 years. The ablated tumors were located variably in either lobe of the liver. Among the baseline characteristics of patients prior to RFA treatment, the cirrhosis stage was significantly more advanced in patients without scattered recurrences (Table 1). Of the patients with scattered recurrences, nine were treated with the cool-tip device according to the original protocol and the other two with the LeVeen needle and original full-expansion method (Tables 1 and 2). After switching from the original to the modified protocols, no scattered recurrences were observed.
| n | Sex | Age | Background | Liver function | Location/ | Device | Protocol | Form of | Interval between RFA | Prognosis |
| (Child-Pugh) | Size (mm) | recurrence | and recurrence (mo) | after RFA (mo) | ||||||
| 1 | F | 75 | LC (HCV) | C | S8/15 | LeVeen | Original | Bilobular | 3 | Death (15) |
| 2 | M | 61 | LC (HBV) | A | S7/20 | Cool-tip | Original | Bilobular | 3 | Alive (42) |
| 3 | F | 79 | LC (HCV) | B | S2/25 | Cool-tip | Original | Bilobular | 2 | Death (36) |
| 4 | M | 63 | C (HBV) | A | S4/15 | Cool-tip | Original | Surrounding | 7 | Death (24) |
| 5 | M | 69 | LC (HCV) | B | S7/26 | Cool-tip | Original | Bilobular | 4 | Death (27) |
| 6 | M | 73 | LC (HCV) | B | S5/13 | Cool-tip | Original | Bilobular | 5 | Death (20) |
| 7 | M | 74 | LC (HCV) | A | S4/18 | Cool-tip | Original | Bilobular | 4 | Death (7) |
| 8 | M | 70 | LC (HCV) | A | S8/30 | Cool-tip | Original | Surrounding | 5 | Alive (36) |
| 9 | M | 70 | LC (HCV) | B | S8/25 | Cool-tip | Original | Bilobular | 6 | Death (16) |
| 10 | M | 51 | LC (HBV) | B | S6/20 | LeVeen | Original | Surrounding | 2 | Death (10) |
| 11 | F | 76 | LC (HCV) | A | S3/14 | Cool-tip | Original | Lobular | 7 | Alive (31) |
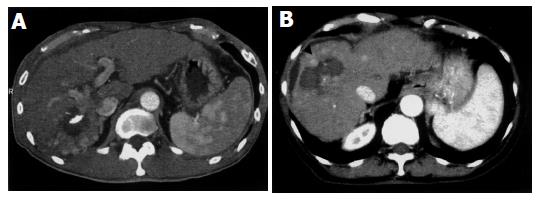
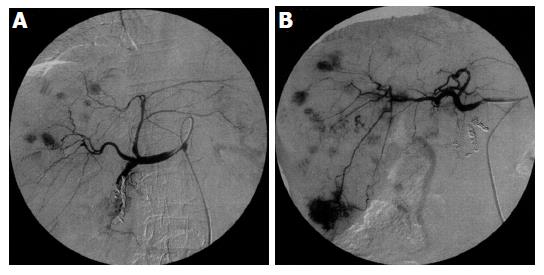
Scattered recurrent tumors occurred in two different patterns. One pattern consisted of scattered tumors that were located in a single lobe (three patients). This pattern is shown in Figures 1A (CT) and 2A (angiography), appearing as multiple tumors around the ablated tumor that were localized in the segment. The other pattern consisted of tumors spread over a single lobule in the several segments (one patient) or both lobules (Figures 1B and 2B) (seven patients). Regardless of the scattering pattern, the tumors were roughly equal in diameter. When recurrences were found, neither tumor thrombus nor extrahepatic metastasis was observed by CT or US imaging.
Follow-up of patients to December 2004 showed that the prognosis was significantly worse for patients with scattered recurrences. Among the 127 patients without scattered recurrences, 40 patients died within the observation period; 8 due to liver failure caused by progression of liver cirrhosis, 2 from variceal rupture, and the remainder due to the progression of HCC. In contrast, 8 of 11 patients with scattered recurrences died and all had advanced HCC.
There are several common characteristics among the cases described in this study. First, recurrence occurred rapidly following RFA. Most of these cases were detected within 6 mo. Second, multiple recurrent tumors were almost equal in diameter. Finally, the recurrent tumors were either scattered around the ablated tumor or all over the lobe(s), and the location had no relation to the puncture route used for RFA.
Nicoli et al[16] recently reported a similar form of recurrence that is, rapid and numerous bilobular recurrent tumors, and proposed that this type of recurrence would result from new communication formed between two vascular regions (arterial and venous-portal) as a result of RFA needle puncture. It was also suggested that the new communication facilitated the migration of tumor cells from a high pressure arterial regions to a low pressure portal liver regions. However, we disagree with this speculation because the tumor treated with RFA in that study was only 3.5 cm in diameter, and the feeding artery would not likely have sufficient pressure to spread the malignant cells throughout the lobes. Ruzzenente et al[17] also identified a series of patients with similar recurrences and suggested that increased intra-tumor pressure might be the cause.
In recent in vitro and in vivo experiments, we demonstr-ated that the pressure in an ablated area can increase drastically during RFA[18]. We assumed that the peculiar scattered recurrence was caused by an explosion due to increased intra-tumor pressure. The explosion could strew the malignant cells, as a large cluster, which would enable the metastatic tumors to grow in a short time.
A substantial increase in pressure would be necessary for the tumor to explode during RFA, and in rider for this to occur either of the two different conditions would be necessary; namely a fibrotic capsule around the tumor, or parenchymal fibrosis surrounding the tumor accompanied by cirrhosis. Without these conditions, the pressure produced by ablation would easily escape through the microvasculature or sinusoids adjacent to the ablated tumor. In our study, all of the patients with scattered recurrences also had liver cirrhosis, and the rate of advanced-stage cirrhosis was higher in patients with recurrence. This suggests that accumulated collagens in the liver of patients with cirrhosis could form a wall that traps in pressure.
Once a scattered recurrence occurs, focal treatment such as ablation therapy can no longer be used, and the only remaining treatment options are TACE or systemic chemotherapy. The incidence of recurrent tumors is also associated with a poor prognosis. We found that within the observation period, the mortality rate of patients with scattered recurrences was higher than that of patients without scattered recurrences.
In order to avoid scattered recurrences, we believe that modified protocols for RFA should be used. After changing to the modified protocols, we found no cases of scattered recurrence associated with either the cool-tip or LeVeen needle procedures. Another way to avoid scattered recurrence is to use alternative procedures to RFA such as ablation by PEIT, which is performed with a thinner needle than that used for RFA and has a lower risk of complications[7,8,20]. There are no reports that we are aware of to indicate that PEIT can cause scattered recurrences. PEIT is generally considered to be inferior to RFA for the following two reasons. First, in contrast with RFA, PEIT requires multiple sessions. Second, local recurrence following PEIT may be more likely when the surgeon is inexperienced, while a highly-skilled surgeon can achieve complete tumor necrosis.
We conclude that critical complications of rapid and scattered recurrences after RFA can be avoided by the use of modified protocols. If the need arises, it is also necessary to select PEIT, not to cling to use RFA procedure. In some cases, PEIT should be considered as a suitable alternative to RFA.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Giorgio A, Francica G, Tarantino L, de Stefano G. Radio-frequency ablation of hepatocellular carcinoma lesions. Radiology. 2001;218:918-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Denys AL, De Baere T, Mahe C, Sabourin JC, Sa Cunha A, Germain S, Roche A. Radio-frequency tissue ablation of the liver: effects of vascular occlusion on lesion diameter and biliary and portal damages in a pig model. Eur Radiol. 2001;11:2102-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Maluf D, Fisher RA, Maroney T, Cotterell A, Fulcher A, Tisnado J, Contos M, Luketic V, Stravitz R, Shiffman M. Non-resective ablation and liver transplantation in patients with cirrhosis and hepatocellular carcinoma (HCC): safety and efficacy. Am J Transplant. 2003;3:312-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Livraghi T. Treatment of hepatocellular carcinoma by interventional methods. Eur Radiol. 2001;11:2207-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 602] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 7. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 879] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 8. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 674] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 9. | Francica G, Marone G, Solbiati L, D'Angelo V, Siani A. Hemobilia, intrahepatic hematoma and acute thrombosis with cavernomatous transformation of the portal vein after percutaneous thermoablation of a liver metastasis. Eur Radiol. 2000;10:926-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Choi H, Loyer EM, DuBrow RA, Kaur H, David CL, Huang S, Curley S, Charnsangavej C. Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics. 2001;21 Spec No:S41-S54. |
| 11. | Dromain C, de Baere T, Elias D, Kuoch V, Ducreux M, Boige V, Petrow P, Roche A, Sigal R. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology. 2002;223:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 933] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 13. | Tamai F, Furuse J, Maru Y, Yoshino M. Intrahepatic pseudoaneurysm: a complication following radio-frequency ablation therapy for hepatocellular carcinoma. Eur J Radiol. 2002;44:40-43. [PubMed] |
| 14. | Koda M, Maeda Y, Matsunaga Y, Mimura K, Murawaki Y, Horie Y. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well-differentiated hepatocellular carcinoma. Hepatol Res. 2003;27:163-167. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol. 2003;8:332-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Nicoli N, Casaril A, Abu Hilal M, Mangiante G, Marchiori L, Ciola M, Invernizzi L, Campagnaro T, Mansueto G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137-1140. [PubMed] |
| 18. | Kotoh K, Nakamuta M, Morizono S, Kohjima M, Arimura E, Fukushima M, Enjoji M, Sakai H, Nawata H. A multi-step, incremental expansion method for radio frequency ablation: optimization of the procedure to prevent increases in intra-tumor pressure and to reduce the ablation time. Liver Int, in press. . |
| 19. | Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004;11:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |