Published online Nov 21, 2005. doi: 10.3748/wjg.v11.i43.6787
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: November 21, 2005
AIM: To assess the changes of portal and arterial velocities, resistance index, spleen and liver size during a long observation period (13.7 years) after orthotopic liver transplantation (OLT).
METHODS: Two hundred and sixty patients were recruited retrospectively for this study and divided into groups with defined time intervals after OLT. The cross-sectional changes of portal and arterial velocities, resistance index, spleen and liver size between the defined time intervals were studied. The complications detected by ultrasound were compared to gold standard methods.
RESULTS: The mean values for liver size were all within the normal range. The splenic size decreased between the time intervals 100 and 1 000 d after OLT (t; P<0.01). While portal and arterial flow velocities decreased up to 5.5 years (t; portal velocity P<0.01, maximal systolic velocity P = 0.05, maximal end diastolic velocity P<0.01), RI increased during this interval (t: P<0.01). Higher RI values were found in older patients (r = 0.24, P<0.001).
CONCLUSION: The arterial and portal velocities show adaptation processes continuing over the course of many years after OLT and are reported for the first time. The vascular complications detected by ultrasound occur mostly up to 100 d after OLT.
- Citation: Boozari B, Gebel M, Bahr MJ, Manns MP, Strassburg CP, Bleck JS, Klempnauer J, Nashan B. Changes of duplex parameters and splenic size in liver transplant recipients during a long period of observation. World J Gastroenterol 2005; 11(43): 6787-6791
- URL: https://www.wjgnet.com/1007-9327/full/v11/i43/6787.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i43.6787
Since the 1980s, OLT has become a standard therapy for patients with end-stage liver disease[1] and till date, the outcome of this therapy depends on the early diagnosis and appropriate treatment of complications[2]. Vascular complications are a frequent cause of early graft failure[3]. Graft damage can be either directly caused by hypoxemia or indirectly by biliary ischemia during graft handling which equally leads to chronic biliary damage[4,5]. Time-dependent changes of splanchnic hemodynamics in liver graft recipients have been reported[6]. Based on these data, it is likely that an understanding and interpretation of splanchnic hemodynamics may lead to the prevention and diagnosis of vascular complications[6].
Apart from physical examination and biochemical work-up, routine and diagnostic imaging procedures are recommended to detect post-OLT vascular complications[7-9]. B-mode and especially color Doppler sonography have played a central role in the monitoring of post-transplantation patients. The use of ultrasound allows for the early diagnosis of hepatic arterial as well as biliary complications[3,9,10] and contributes to a better understanding of splanchnic hemodynamic changes[6].
Long-term follow-up studies of splanchnic hemodynamic changes after OLT are time consuming and therefore not applicable. The aim of this study was to assess the post-transplantation changes of duplex parameter as well as liver and splenic size in liver graft recipients for a long period of observation. This study was performed retrospectively as a cross-sectional analysis in a large cohort of OLT patients. The secondary objective of this study was to assess the post-transplantation complications detected by ultrasound during this observation time.
All the liver graft recipients received routine ultrasound examinations in our sonography department. For this study, we chose a sample of all OLT patients (with or without clinical problems) who received ultrasound examinations between February 2000 and December 2001. Five hundred and eighteen ultrasound examinations on 282 patients were performed during this period. Patients who received at least one ultrasound examination after liver transplantation were included in this study. In the case of multiple ultrasound examinations, one examination was chosen randomly in order to prevent dependencies, due to multiple measurements of a single case. Patients who had acute cardiac or renal failure were excluded (n = 2). Two hundred and sixty consecutive patients were included, and their clinical characteristics are shown in Tables 1 and 2.
| Parameter | Value |
| Gender (M/F) | 165/95 |
| Age (yr) | 49.8±0.8 |
| Age (yr) | 18.5–74.5 |
| Mean time after OLT (d) | 1 523.3±97.6 |
| Mean US evaluation time after OLT (d) | 2–8 912 |
| Full size OLT (n) | 244 |
| Split liver OLT (n) | 16 |
| Etiology | Cases (n) |
| Chronic hepatitis B | 37 |
| Primary sclerosing cholangitis | 36 |
| Chronic hepatitis C | 33 |
| Chronic hepatitis and hepatocellular carcinoma | 24 |
| Primary biliary cirrhosis | 18 |
| Cryptogenic liver disease | 18 |
| Cystic liver degeneration | 12 |
| Autoimmune hepatitis | 12 |
| Budd-Chiari syndrome | 12 |
| Alcoholic cirrhosis | 10 |
| Primary hepatocellular carcinoma | 8 |
| Wilson’s disease | 8 |
| Others1 | 32 |
All patients were examined with high-end ultrasound equipment Power Vision 8000 (Toshiba, Japan) and Elegra Sonoline Advanced (Siemens, Germany) using convex arrays 3.5C40H (Siemens, Germany) and C 3-6 MHz (Toshiba, Japan) as well as sector array 3-6 MHz (Toshiba, Japan). The patients were examined by two gastroenterologists with more than 20 years of experience in the field of ultrasound.
Systematic B-mode examination of all abdominal organs including the retroperitoneum was performed following the recommendations of the German Association of Ultrasound in Medicine (DEGUM). Liver size was measured by the diameter of the right lobe in the mid clavicular line (MCL). The spleen length was measured from upper to lower pole in an oblique intercostal array position. The normal liver size was defined as 13±0.5 cm[11]. The normal spleen size was defined as 11±0.5 cm[11]. In addition, the portal vein and the bile duct anastomosis were examined in the proximal and distal portion.
The maximum velocity of the portal vein [(P)Vmax], the maximum systolic velocity [(A)Vmax], the maximum end diastolic velocity [(A)Vmin] and the resistance index of the hepatic artery (RI) were measured before and after the anastomosis in each case after an overnight fasting. Settings such as gain, filter, and pulse-repetition frequencies were adjusted as needed for optimal signal detection to prevent artifacts. Segmental arterial stenosis was considered to be present when circumscript aliasing was visualized by color Doppler sonography using the maximal pulse repetition frequency (PRF) as well as the presence of a Vmax exceeding 170 cm/s. Alternatively, in case of the absence of a segmental stenosis, the detection of a tardus-parvus duplex spectrum with a cut off RI below 0.5 in addition to a Vmax>170 cm/s was required for the definition of a stenosis. Stenosis of the portal vein required a two-fold increase of flow velocity in the stenosis. Dilated intrahepatic and extrahepatic bile ducts were defined as exceeding 3 and 10 mm in diameter (DEGUM guidelines), respectively.
The complications detected by ultrasound were confirmed by following the gold standard methods. In all patients with suspected stenosis or thrombosis of the hepatic artery by ultrasound examination, a CT-angiography was performed to confirm the diagnosis. Most of the biliary complications such as bile duct dilatation, thickening, stricture, and calculi in the bile duct system were confirmed by endoscopic retrograde cholangiopancreaticography (ERCP) in patients with chocholedochostomy[12] and by percutaneous transhepatic cholangiography (PTC) in patients with status after hepaticojejunostomy. The occurrence of intrahepatic abscesses was confirmed by biopsy. Liver biopsies after OLT were available from 94 patients (n = 45 acute rejections, n = 2 chronic rejections, n = 2 ischemia, n = 21 viral re-infection, n = 21 fibrosis, n = 7 cirrhosis, n = 33 cholangitis).
Statistical evaluation was performed using the SPSS 11.5 software package for Windows™. Mean values and standard errors of the means (mean±SE) as well as frequencies were calculated. Correlations were done using Spearman’s (S) rank correlation coefficient. We also analyzed the changes of the velocity values as well as organ sizes in the course of time. Due to different time points of ultrasound examinations after OLT, we divided the patients in groups with defined time intervals after OLT. The time points were days 100 (1), 1 000 (2), 2 000 (3), 3 000 (4), 4 000 (5) and 5 000 (6) after OLT. The number of patients at the defined time intervals was as follows: n = 20 (1), n = 120 (2), n = 81 (3), n = 48 (4), n = 25 (5), n = 11 (6). At these time points, the data of patients before the defined time point (days after transplantation) were compared with the data of patients after this time point. Mean values of the parameters were calculated and compared for significance using the t-test. Therefore, our data reflected only the changes of the studied parameters at these time points. The changes of the parameters in the group of patients before the defined time points were graphically demonstrated. The graphs were assembled using the Prism 3.0 software package.
Correlations as well as the mean values were calculated with and without extremes. The extremes were defined as stenosis or thrombosis of the portal vein and/or the hepatic artery (n = 8).
Vascular complications over a 13.7-year observation period were detected in one case (0.4%) of thrombosis and five cases (1.9%) of stenosis of the hepatic artery and in seven cases (2.7%) of stenosis of the portal vein by ultrasound (Table 3). All cases were confirmed by CT angiography.
| US finding | US frequency in this study (%) | Reported frequency (%) |
| Thrombosis of the hepatic artery | 0.4 | 12 [21] |
| Stenosis of the hepatic artery | 1.9 | 3–5 [8] |
| Stenosis of the portal vein | 2.7 | 1–6.2 [3,17] |
| Biliary complications generally | 22.3 | 18 [22] |
| Bile duct dilatations | 17.7 | 7.3–48.8 [20] |
| Thickening of bile ducts | 6.9 | |
| Abscess | 0.8 | |
| Calculi of biliary system | 6.2 | 36.6 [20] |
| Bile duct stricture | 0.4 | 5–14 [21] |
| Ascites | 5.4 | |
| Splenomegaly | 43.8 |
In a 13.7-year observation period by ultrasound examination, 58 (22.3%) patients showed biliary complications, of them 33 (56.9%) had either ERCP or PTC. In 29 patients (87.9%), ultrasound diagnosis was confirmed either by ERCP or PTC. Nine patients (3.5%) had extrahepatic dilatation and 37 cases (14.2%) had intrahepatic dilatation of the bile ducts. One patient (0.4%) had calculi, 14 (5.4%) patients had sludge in the intra- and extra-hepatic bile ducts. Eighteen patients (6.9%) showed a thickening of intra- and extra-hepatic bile duct walls, two cases (0.8%) had intrahepatic abscesses, one patient (0.4%) had stricture of the main hepatic bile duct, 114 patients (43.8%) had splenomegaly after OLT, and 14 patients (5.4%) had detectable ascites (Table 3).
The mean liver size after OLT was 12.2±0.2 cm in MCL. The mean splenic size after liver transplantation was 12.9±0.2 cm (Table 4). Women had a smaller spleen than men (12.2±0.3 cm vs 13.4±0.2 cm, t; P<0.01).
| Parameter | Value | Unit |
| (P)Vmax | 30.0±1.5 | cm/s |
| (A)Vmax | 67.1±4.2 | cm/s |
| (A)Vmin | 20.4±1.6 | cm/s |
| RI | 0.69±0.01 | |
| Spleen size | 12.9±0.2 | cm |
| Liver size in MCL | 12.2±0.2 | cm |
The changes of mean values for liver and splenic size 100, 1 000, 2 000, 3 000, 4 000, and 5 000 d after OLT were calculated. The mean values for liver size after OLT were all within the normal range and did not change significantly between the studied time intervals. In contrast, the splenic size decreased between the intervals 100 and 1 000 d after OLT. After this interval, the splenic size increased. Consequently, the spleens in the patients more than 1 000 d after OLT were significantly larger than those in the patients less than 1 000 d after OLT (13.6±0.3 cm vs 12.2±0.3 cm, t; P<0.01). The mean splenic size remained higher than the normal range throughout the observed time in this study.
The mean value of color Doppler data for all patients is shown in Table 4. There was an inverse correlation between (P)Vmax and time after OLT (r = -0.41, P<0.001). After exclusion of patients with stenosis and thrombosis of the arterial and portal vein anastomosis in order to eliminate the velocity extremes, the correlation coefficients still remained high (r = 0.38, P<0.001). There was also an inverse correlation between (A)Vmax and (A)Vmin of the hepatic artery and time after OLT [for (A)Vmaxr = 0.21, P<0.01 and for (A)Vminr = 0.23, P<0.01]. After exclusion of extremes, the correlation persisted [for (A)Vmaxr = 0.21, P<0.01 and for (A)Vminr = 0.22, P<0.01]. We could not observe a correlation between RI and time after OLT, but we confirmed a significant correlation between RI and age of the patients (r = 0.24, P<0.001).
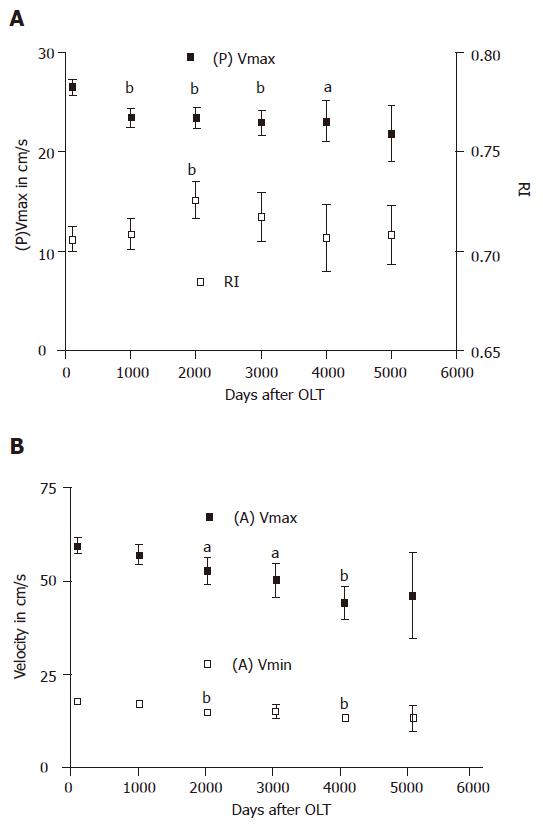
The changes of mean values of all color Doppler parameters were calculated 100, 1 000, 2 000, 3 000, 4 000, and 5 000 d post OLT after exclusion of extremes. (P)Vmax decreased between the time points 100 and 3 000 d after OLT. It was stabilized at a level of 23 cm/s between the time points 3 000 and 4 000 d after OLT and dropped again from day 4 000 (Figure 1A). (A)Vmax decreased from 100 up to 4 000 d after OLT (Figure 1B). (A)Vmin decreased from 2 000 d after OLT (Figure 1B). RI increased between 100 and 2 000 d after OLT. From this time point on, we observed a decrease of RI which was stabilized at a level of 0.70 from time point 4 000 d after OLT to the end of the observation time (Figure 1A).
All vascular complications detected by duplex measure-ments in this study occurred within the first 100 d after liver transplantation (Table 5). Due to this information as well as the fact that complications such as rejection appear mostly during the early phase after OLT, drastic long-term hemodynamic changes in these patients are not expected. All vascular complications were initially diagnosed by ultrasound examination. Interestingly, a satisfactory blood flow was observed intraoperatively, but these patients developed stenosis after OLT. Routine and protocol ultrasound examinations during this interval are therefore recommended[13,14]. The flow characteristics of patients early after OLT have been reported in prospective studies[6,15]. We therefore focused our study on the long-term changes of these parameters.
| Arterial system | |
| Reperfusion damage | (n = 2) |
| Thrombosis of the celiac trunk | (n = 1) |
| Thrombosis of the hepatic artery1 | (n = 1) |
| Dissection of the common hepatic artery | (n = 1) |
| Unknown | (n = 1) |
| Portal system | |
| Over average length of the portal anastomosis | (n = 3) |
| Intraoperative thrombectomy2 | (n = 2) |
| Intraoperative thrombectomy of malign thrombus3 | (n = 1) |
| Leakage of the biliary anastomosis and consequently | (n = 1) |
| Systemic infection leading to portal thrombosis |
The long-term hemodynamic changes in these patients seem to be influenced by factors in the graft itself or alteration of the vascular track. The prospective study of Bolognesi et al[6] is the largest follow-up study investigating the hemodynamic changes in patients after OLT. Compared to this study, our study showed a more heterogeneous spectrum of the underlying liver diseases. Our findings showed that the mean liver size remained within the normal range, independent of the complications, even many years after OLT. Based on the results of liver biopsies after OLT, at least 20% of the patients included in this study had parenchymal changes such as cirrhosis or fibrosis which consequently leads to a smaller liver size. Interestingly, the mean values of liver size still remained stable in the course of studied time points. The mean splenic size was smaller than that of previously reported[6] which may be explained by the heterogeneity of liver diseases with a considerable amount of transplantations without portal hypertension.
Depending on the evaluation time after OLT, an initial increase in portal blood flow in patients with cirrhosis has been reported, which was normalized within 2 years[6]. We observed a decrease of portal blood flow from 100 d to 8.2 years after OLT. This stable decrease may also be influenced by the low prevalence of portal stenosis and thrombosis in our patients. We also detected a decrease of arterial velocities over a long period of time after OLT. This could be caused by the normalization of hyperdynamic circulatory syndromes of patients with cirrhosis at the time of OLT. While the role of arterial RI in patients after kidney transplantation has been extensively studied, the RI changes in liver graft recipients are still unclear. It was reported that a high arterial RI after kidney transplantation is associated with poor subsequent allograft performance and death[16]. In liver graft recipients, an early increase in hepatic arterial resistance has been reported[6,17], which is related to older donor age and prolonged period of ischemia. Higher RI values were found in older patients in our study. RI increase is also attributed to the elevation of portal blood flow early after OLT[6]. In our study, RI did not correlate with portal flow.
Our data have confirmed the reported rate of ultrasound detected complications in patients after liver transplantation. However, the sensitivity of ultrasound for detection of biliary complications is better than that of previously reported[12]. Arterial thrombosis after transplantation has an estimated incidence of 12%[8]. In our patients, thrombosis of the hepatic artery was detected by ultrasound in 0.4% of cases. Since the majority of arterial thromboses occur during the early post-transplantation period[3] and most of our examinations were performed 100 or more days after OLT, the low prevalence of arterial thrombosis in this study may be related to the time point of investigation. The detection rate of hepatic artery stenosis as well as portal vein thrombosis in our study was comparable to the reported incidence[8,18,7]. The detection rate of biliary tract complications in this study (22.3%) is higher than the reported incidence approaching 18%[19]. Regarding the cases, which were confirmed by ERCP or PTC in our study, ultrasound has a sensitivity of 88%[12].
This study provides an insight into time point of vascular complications as well as hemodynamic and parenchymal adaptive processes present in liver graft recipients. The cross sectional characteristic of our study did not allow a continuous assessment of hemodynamic changes in the course of time, but it enables the analysis of a very long observation period. These data can serve as an orientation for the examining physicians to have a better understanding and interpretation of duplex changes in these patients.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Nashan B, Lück R, Becker T, Grannas G, Strassburg C, Schneider A, Melter M, Strassburg A, Klempnauer J. Expansion of the donor pool in liver transplantation: the Hannover experience 1996-2002. Clin Transpl. 2002;221-228. [PubMed] |
| 2. | Brems JJ, Hiatt JR, Colonna JO, el-Khoury G, Quiñones WJ, Ramming KP, Ziomek S, Busuttil RW. Variables influencing the outcome following orthotopic liver transplantation. Arch Surg. 1987;122:1109-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | De Gaetano AM, Cotroneo AR, Maresca G, Di Stasi C, Evangelisti R, Gui B, Agnes S. Color Doppler sonography in the diagnosis and monitoring of arterial complications after liver transplantation. J Clin Ultrasound. 2000;28:373-380. [PubMed] [DOI] [Full Text] |
| 4. | Langnas AN, Marujo W, Stratta RJ, Wood RP, Shaw BW. Vascular complications after orthotopic liver transplantation. Am J Surg. 1991;161:76-82; discussion 82-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 325] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 357] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Bolognesi M, Sacerdoti D, Bombonato G, Merkel C, Sartori G, Merenda R, Nava V, Angeli P, Feltracco P, Gatta A. Change in portal flow after liver transplantation: effect on hepatic arterial resistance indices and role of spleen size. Hepatology. 2002;35:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Kok T, Boeve WJ, Prins TR, Baarslag HJ, Woesthuis M, Slooff MJ, Haagsma EB, Bijleveld CM, van der Jagt EJ. Arteriography and portal venography on routine follow-up after orthotopic liver transplantation. Invest Radiol. 2000;35:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Pinna AD, Smith CV, Furukawa H, Starzl TE, Fung JJ. Urgent revascularization of liver allografts after early hepatic artery thrombosis. Transplantation. 1996;62:1584-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Dalgic A, Dalgic B, Demirogullari B, Ozbay F, Latifoglu O, Ersoy E, Mahli A, Ilgit E, Ozdemir H, Arac M. Clinical approach to graft hepatic artery thrombosis following living related liver transplantation. Pediatr Transplant. 2003;7:149-152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Shaw AS, Ryan SM, Beese RC, Sidhu PS. Ultrasound of non-vascular complications in the post liver transplant patient. Clin Radiol. 2003;58:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Schmidt G. Ultraschall Kursbuch. 4 ed. Stuttgart: Thieme Verlag 2003; . |
| 12. | Hussaini SH, Sheridan MB, Davies M. The predictive value of transabdominal ultrasonography in the diagnosis of biliary tract complications after orthotopic liver transplantation. Gut. 1999;45:900-903. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Heffron TG, Emond JC, Whitington PF, Thistlethwaite JR, Stevens L, Piper J, Whitington S, Broelsch CE. Biliary complications in pediatric liver transplantation. A comparison of reduced-size and whole grafts. Transplantation. 1992;53:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 105] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Nishida S, Kato T, Levi D, Naveen M, Thierry B, Vianna R, Selvaggi G, Buitorago E, Al-Niami A, Nakamura N. Effect of protocol Doppler ultrasonography and urgent revascularization on early hepatic artery thrombosis after pediatric liver transplantation. Arch Surg. 2002;137:1279-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Piscaglia F, Zironi G, Gaiani S, Mazziotti A, Cavallari A, Gramantieri L, Valgimigli M, Bolondi L. Systemic and splanchnic hemodynamic changes after liver transplantation for cirrhosis: a long-term prospective study. Hepatology. 1999;30:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Radermacher J, Mengel M, Ellis S, Stuht S, Hiss M, Schwarz A, Eisenberger U, Burg M, Luft FC, Gwinner W. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 17. | García-Criado A, Gilabert R, Salmerón JM, Nicolau C, Vilana R, Bianchi L, Buñesch L, García-Valdecasas JC, Rimola A, Brú C. Significance of and contributing factors for a high resistive index on Doppler sonography of the hepatic artery immediately after surgery: prognostic implications for liver transplant recipients. AJR Am J Roentgenol. 2003;181:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Molmenti EP, Levy MF, Molmenti H, Casey D, Fasola CG, Hamilton WM, Jung G, Marubashi S, Gogel BM, Goldstein RM. Correlation between intraoperative blood flows and hepatic artery strictures in liver transplantation. Liver Transpl. 2002;8:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Gomez R, Moreno E, Castellon C, Gonzalez-Pinto I, Loinaz C, Garcia I. Choledochocholedochostomy conversion to hepaticojejunostomy due to biliary obstruction in liver transplantation. World J Surg. 2001;25:1308-1312. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Huang DZ, Le GR, Zhang QP, Li KY, Ye QF, Zhu W, Chen YC. The value of color Doppler ultrasonography in monitoring normal orthotopic liver transplantation and postoperative complications. Hepatobiliary Pancreat Dis Int. 2003;2:54-58. [PubMed] |
| 21. | Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, Wiesner RH, Krom RA. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 291] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Busuttil RW, Goldstein LI, Danovitch GM, Ament ME, Memsic LD. Liver transplantation today. Ann Intern Med. 1986;104:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Wolfsen HC, Porayko MK, Hughes RH, Gostout CJ, Krom RA, Wiesner RH. Role of endoscopic retrograde cholangiopancreatography after orthotopic liver transplantation. Am J Gastroenterol. 1992;87:955-960. [PubMed] |