Published online Nov 21, 2005. doi: 10.3748/wjg.v11.i43.6775
Revised: April 26, 2005
Accepted: April 30, 2005
Published online: November 21, 2005
AIM: To detect the DNA binding activity of nuclear factor-kappaB (NF-кB) in rat hepatocyte and to investigate the effects of NF-кB on rat hepatocyte regeneration and apoptosis after 70% portal branch ligation.
METHODS: Sixty Wistar rats were randomly divided into control group and portal branch ligation group. The animals were killed 12 h, 1, 2, 3, 7, and 14 d after surgery to determine the contents of plasma ALT. Hepatocytes were isolated and nuclear protein was extracted. DNA binding activity of NF-κB was measured by EMSA. Hepatocyte regeneration and apoptosis were observed under microscope by TUNEL staining. The ultrastructural changes of liver were observed under electron microscope.
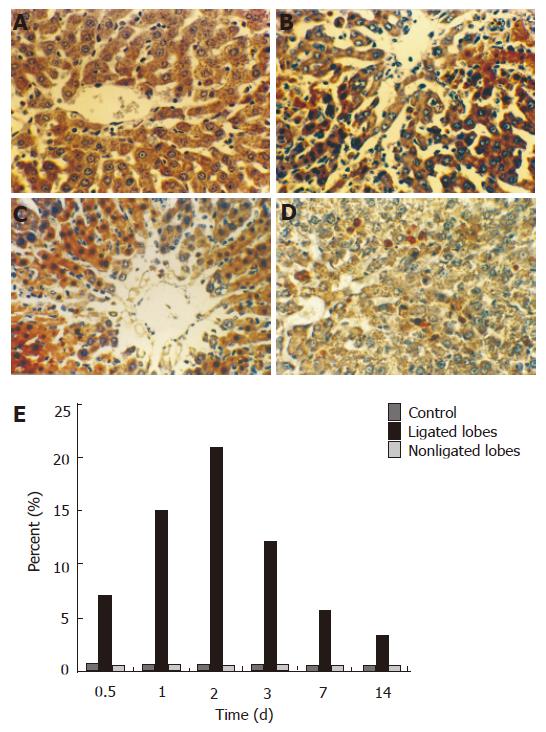
RESULTS: Seventy percent portal branch ligation produced atrophy of the ligated lobes and the perfused lobes underwent compensatory regeneration, the total liver weight and plasma ALT levels were maintained at the level of sham-operated animals throughout the experiment. After 2 d of portal branch ligation, DNA binding activity of NF-кB in hepatocyte increased and reached its peak, the number of apoptotic hepatocyte in the ligated lobes and the number of mitotic hepatocyte in the perfused lobes also reached their peak. Typical apoptotic changes and evident fibrotic changes in the ligated lobes were observed under electron microscope.
CONCLUSION: After 70% portal branch ligation, DNA binding activity of NF-кB in hepatocyte is significantly increased and NF-кB plays an important role in hepatocyte regeneration and apoptosis.
- Citation: Yang WJ, Zhang QY, Yu ZP, Song QT, Liang HP, Xu X, Zhu GB, Jiang FZ, Shi HQ. Effects of nuclear factor-kappaB on rat hepatocyte regeneration and apoptosis after 70% portal branch ligation. World J Gastroenterol 2005; 11(43): 6775-6779
- URL: https://www.wjgnet.com/1007-9327/full/v11/i43/6775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i43.6775
Portal branch ligation (PBL) or embolization is widely used in the treatment of liver carcinoma, especially in the treatment of patients who have already missed the surgical opportunity[1-3]. PBL or embolization could produce atrophy of the ligated lobes and the perfused lobes undergo compensatory regeneration, while the liver structure and function maintained normal. But the mechanism is still unclear.
It is demonstrated that nuclear factor-kappaB (NF-κB) plays an important role in cell regeneration and apoptosis after partial hepatectomy[4-6]. To study its effects on hepatocyte regeneration and apoptosis, we observed the changes of DNA binding activity of NF-κB in rat liver and its relations to hepatocyte regeneration and apoptosis after 70% PBL.
Sixty Wistar rats, weighing 200-240 g, were obtained from the Animal Center of the Third Military University and used in all experiments. All animals were kept in a temperature- and humidity-controlled environment in a 12-h light/dark cycle and allowed free access to water and standard food-pellet diet.
All surgical procedures were carried out under sodium pentobarbital (40 mg/kg intraperitoneally) anesthesia at room air between 9:00 and 12:00 a.m. with a clean but not sterile technique. The 70% PBL model used was based on the Bilodeau method[7]. In 70% PBL, a median laparotomy was performed, and the branch of the portal vein feeding the anterior and lateral lobes was carefully dissected under an operating microscope and completely ligated with a 7-0 suture. Care was taken not to injure the hepatic artery and the bile duct and to avoid hemorrhage. In sham-operated rats, a laparotomy followed by dissection of the relevant ligaments without ligature was performed. The animals had free access to water and food after surgery. The animals were killed 12 h, 1, 2, 3, 7, and 14 d after surgery.
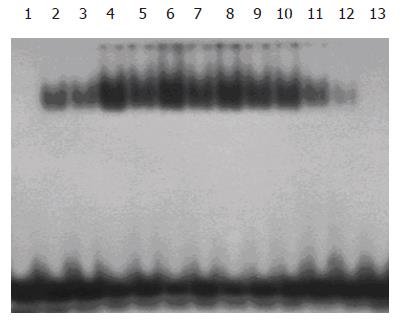
Nuclear extracts were prepared separately from the anterior (ligated) and posterior (nonligated) lobes as previously described[8]. Protein concentration was determined using the Bradford method. Double-stranded NF-κB consensus oligonucleotides (5’-AGT TGA GGG GAC TTT CCC AGG C-3’, 3’-TCA ACT CCC CTG AAA GGG TCC G-5’, Promega Co., USA) were end-labeled[5] with [γ-32P] ATP (Beijing Yahui Biomed Inc., Beijing, China) using T4 polynucleotide kinase (Promega Co.). After the probe was purified, 5 µg of nuclear proteins was preincubated for 10 min at room temperature with 2 µg poly (dI-dC) (Sigma Co., USA) in the binding buffer. Double-stranded oligonucleotides were 32P end-labeled with [γ-32P] ATP and added to the extracts. The mixture was further incubated for 30 min at room temperature and then electrophoresed (200 V, 2 h) on a 5% polyacrylamide gel in a 0.5× TBE buffer. Then the gel was subjected to gamma autoradiography at -70 °C for 12 h, and analyzed with gel imaging system (Biorad Co., USA).
The rat liver color and quality were observed. Both ligated and nonligated lobes were weighed separately for measurement of their absolute and relative weights (the ratio of liver weight/body weight), and their percent in the whole liver was calculated.
Serum ALT level was measured with the biochemical multi-analyzer in Biochemistry Department of our hospital.
Liver sections were derived from formaldehyde-fixed tissues embedded in paraffin and stained with hematoxylin–phloxin–saffron. Mitotic activity in stained sections was determined. Mitotic hepatocytes were sought in 100 consecutive high-power fields (×400), and mitotic index was expressed as per 1 000 hepatic nuclei. Prophases before dissolution of nuclear membrane and late telophases were excluded.
Tissue sections of rat liver were dewaxed in toluene and alcohol. After rehydration with phosphate-buffered saline, they were incubated with proteinase K (25 μg/mL) for 15 min at room temperature and then with terminal deoxyribonucleotidyl transferase (1 U/mL) and digoxigenin-tagged dUTP (10 μmol/L) at 37 °C for 1 h in 0.2 mol/L sodium cacodylate, 30 mmol/L Tris (pH 7.2), 1 mmol/L CoCl2, and 0.25 mg/mL bovine serum albumin. The sections were exposed to anti-digoxigenin antibodies labeled with alkaline phosphatase for 30 min at room temperature in 50 mmol/L Tris (pH 7.4) and 150 mmol/L NaCl. The binding of the antibody was revealed with 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphate reagents. Slides were counterstained with 1% eosin.
The rat liver was cut into sections as large as 1 mm×1 mm×1 mm, which were embedded in Epon618 resin and stained with uranyl acetate and lead citrate. H-2000 transmission electron microscope was used to study the ultrastructure.
Results were expressed as mean±SD. The statistical difference between the groups was tested using the one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
After 12 h of 70% PBL, DNA binding activity of NF-κB both in ligated and nonligated lobes increased and reached its peak on d 2, and returned to normal on d 7. The changes of DNA binding activity of NF-κB in the ligated lobes were more obvious than those in the perfused lobes. No NF-κB binding was observed in nuclear extracts from control animals (Figure 1).
The liver lobes deprived of portal flow were darkened, rapid and progressive atrophy occurred, with their proportion being reduced gradually in the whole liver. At the same time, the nonligated lobes were progressively enlarged, the total liver weight was maintained at the level of sham-PBL controls at each time interval (Figure 2A).
There were no changes of serum ALT level in control group. After 70% PBL, the serum ALT level only increased slightly 1 d after surgery and then returned to normal (Table 1).
| Group | 0.5 d | 1 d | 2 d | 3 d | 7 d | 14 d |
| Control | 55.6±8.9 | 43.7±10.4 | 38.5±7.8 | 40.1±9.4 | 36.4±11.3 | 42.0±13.7 |
| PBL | 59.7±17.3 | 67.9±15.7a | 44.6±9.8 | 42.6±7.8 | 38.9±12.1 | 41.3±7.9 |
No apparent changes were found at light microscopic examination in the control liver. One day after 70% PBL, mild necrosis was found in the ligated lobes, mainly around the central vein. The cytoplasm was homogenous, and the nuclear pyknosis inside the necrosis and neutrophils was seen in some lesions. After 3 d, the necrosis was partly resorbed, and many monocytes were seen inside them. The lobules were small, with portal areas lying near each other. One to two weeks after ligation, the necrosis was completely disappeared, and the lobules became small. Fibrosis appeared around larger portal and hepatic veins, and bile ducts were collapsed with low epithelium.
Remarkable hepatocyte regeneration was observed in the nonligated lobes 12 h after 70% PBL and reached its peak 2 d later, and was still high after 7 d. The liver structure was almost normal (Figure 2B).
There were only few apoptotic hepatocytes in the liver of control animals and in the nonligated lobes after 70% PBL. Many apoptotic hepatocytes were found in the ligated lobes 1 d after 70% portal branch ligation, and reached its peak 2 d later. These cells were mainly present around the central veins with necrosis (Figures 3A-E).
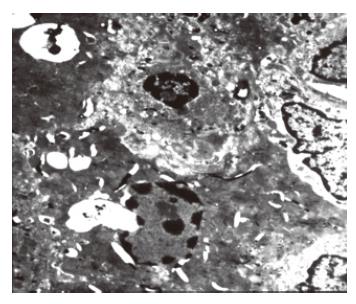
The liver ultrastructure was normal in control animals and was almost normal in the ligated lobes early after 70% PBL, and only wild necrosis was found in some local areas. One day after 70% PBL, many apoptotic hepatocytes were found in the ligated lobes. Histological evidence for apoptosis included disappearance of the nuclear membrane, condensation, and margin of karyoplasms or chromatin, pieces of nuclei. There were no morphological changes in the mitochondriae and other intracellular structures (Figure 4). A number of apoptotic hepatocytes reached its peak 2 d later. Collagen was deposited in the Disse space and hepatic sinus became narrow 7 d after 70% PBL. Evident fibrotic changes were found in the ligated lobes 14 d after 70% PBL. A lot of collagens were deposited in the Disse space and portal areas, between hepatocytes and in hepatocytes.
PBL or embolization is widely used in the treatment of liver carcinoma, especially in the treatment of patients who have missed the opportunity of surgery[1-3]. It was verified in our experiment that PBL could produce atrophy of the ligated lobes, whereas the perfused lobes underwent compensatory regeneration, the liver structure and function maintained normal. Therefore, it is safe and practicable to ligate 70% portal vein branch in normal rat liver.
The 70% PBL could produce atrophy of the ligated lobes through hepatocyte apoptosis; whereas the perfused lobes undergo compensatory regeneration through hepatocyte mitosis. The total liver weight and function maintained normal. The mechanism of rat liver is still unclear. We observed the changes of DNA binding activity of NF-κB in liver through EMSA. After 70% PBL, DNA binding activity of NF-κB significantly increased both in ligated lobes and nonligated lobes, which was positively correlated with hepatocyte regeneration and apoptosis. Therefore, we could conclude that NF-κB plays an important role in hepatocyte regeneration and apoptosis after 70% PBL.
NF-κB, as a universal nuclear transcriptional factor, plays an important role in the regulation of genes relative to cell regeneration and apoptosis[9,10]. In most cells, NF-κB heterodimers are present in the cytoplasm forming an active complex by interacting with the IкB family of proteins. In response to a variety of activators, IкB-α, the prototypic member of this family of inhibitors, is phosphorylated at series 32 and 36, rendering the factor susceptible to proteolysis via the ubiquitin–proteasome pathway. This event unmasks a nuclear localization sequence of the transactivating heterodimers, allowing NF-κB translocation to nuclei. Therefore, the complex binds to кB consensus motifs in DNA, upregulating the transcription of many genes[11-14].
NF-κB plays an important role on hepatocyte regeneration in nonligated lobes by inhibiting hepatocyte apoptosis and accelerating hepatocyte regeneration. NF-κB can inhibit hepatocyte apoptosis by regulating relative cytokine transcription and expression[15-17], inducing antiapoptotic genes in Bcl-2 family[18], regulating the expression of TRAF and IAP at transcription and translation level and inhibiting the activation of caspase-8, a key enzyme in cell apoptosis[19-21], activating the apoptotic inhibitors[22,23]. NF-κB, which takes part in hepatocyte regeneration, may be mediated by regulating the transcription and expression of relative genes. NF-κB can activate the transcription and expression of TNF-α, increased TNF-α can stimulate secretion of IL-6, which can activate STAT3 through combining with IL-6R on the surface of hepatocyte, and accelerate hepatocyte regeneration[24-27].
We observed that the changes of DNA binding activity of NF-κB in the ligated lobes were more obvious than those in the perfused lobes. One reason is that compensatory response to the excessive hepatocyte apoptosis could maintain the relative weight and function of the liver. The other reason is that NF-κB plays an important role in accelerating hepatocyte apoptosis. Kuhnel et al[28] reported that NF-κB mediates hepatocyte apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. Recently the induction of NF-κB through the double-stranded RNA-dependent protein kinase has been suggested as a principal mechanism of virus-mediated apoptotic cell death[29].
In conclusion, NF-κB plays a completely different role in the ligated lobes and nonligated lobes through different stimulating factors and different signal transduction pathways. This may have important significance in maintaining liver structure and function after 70% PBL.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Tanaka H, Hirohashi K, Kubo S, Shuto T, Higaki I, Kinoshita H. Preoperative portal vein embolization improves prognosis after right hepatectomy for hepatocellular carcinoma in patients with impaired hepatic function. Br J Surg. 2000;87:879-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Hatsuno T, Kaneko T, Inoue S, Sugimoto H, Takeda S, Nakao A. Changes in hepatic lobe volume in hepatocellular carcinoma after transcatheter arterial and percutaneous transhepatic portal embolization. Hepatogastroenterology. 2004;51:1820-1824. [PubMed] |
| 3. | Kokudo N, Makuuchi M. Current role of portal vein embolization/hepatic artery chemoembolization. Surg Clin North Am. 2004;84:643-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Riggins RB, Zwart A, Nehra R, Clarke R. The nuclear factor kappa B inhibitor parthenolide restores ICI 182,780 (Faslodex; fulvestrant)-induced apoptosis in antiestrogen-resistant breast cancer cells. Mol Cancer Ther. 2005;4:33-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Singh RP, Mallikarjuna GU, Sharma G, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin Cancer Res. 2004;10:8641-8647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Djavaheri-Mergny M, Javelaud D, Wietzerbin J, Besançon F. NF-kappaB activation prevents apoptotic oxidative stress via an increase of both thioredoxin and MnSOD levels in TNFalpha-treated Ewing sarcoma cells. FEBS Lett. 2004;578:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Bilodeau M, Aubry MC, Houle R, Burnes PN, Ethier C. Evaluation of hepatocyte injury following partial ligation of the left portal vein. J Hepatol. 1999;30:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Kawamura I, Morishita R, Tsujimoto S, Manda T, Tomoi M, Tomita N, Goto T, Ogihara T, Kaneda Y. Intravenous injection of oligodeoxynucleotides to the NF-kappaB binding site inhibits hepatic metastasis of M5076 reticulosarcoma in mice. Gene Ther. 2001;8:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Tang JB, Xu Y, Wang XT. Tendon healing in vitro: activation of NIK, IKKalpha, IKKbeta, and NF- kappaB genes in signal pathway and proliferation of tenocytes. Plast Reconstr Surg. 2004;113:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Sánchez A, Factor VM, Schroeder IS, Nagy P, Thorgeirsson SS. Activation of NF-kappaB and STAT3 in rat oval cells during 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Hepatology. 2004;39:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Buss H, Dörrie A, Schmitz ML, Frank R, Livingstone M, Resch K, Kracht M. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem. 2004;279:49571-49574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Natarajan R, Fisher BJ, Jones DG, Fowler AA. Atypical mechanism of NF-kappaB activation during reoxygenation stress in microvascular endothelium: a role for tyrosine kinases. Free Radic Biol Med. 2002;33:962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2972] [Cited by in RCA: 3213] [Article Influence: 139.7] [Reference Citation Analysis (0)] |
| 14. | Schooley K, Zhu P, Dower SK, Qwarnström EE. Regulation of nuclear translocation of nuclear factor-kappaB relA: evidence for complex dynamics at the single-cell level. Biochem J. 2003;369:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Zong WX, Edelstein LC, Chen C, Bash J, Gélinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 571] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 16. | Shah RD, Gonzales F, Golez E, Augustin D, Caudillo S, Abbott A, Morello J, McDonough PM, Paolini PJ, Shubeita HE. The antidiabetic agent rosiglitazone upregulates SERCA2 and enhances TNF-alpha- and LPS-induced NF-kappaB-dependent transcription and TNF-alpha-induced IL-6 secretion in ventricular myocytes. Cell Physiol Biochem. 2005;15:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Takada I, Suzawa M, Kato S. Nuclear receptors as targets for drug development: crosstalk between peroxisome proliferator-activated receptor gamma and cytokines in bone marrow-derived mesenchymal stem cells. J Pharmacol Sci. 2005;97:184-189. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Wang CY, Guttridge DC, Mayo MW, Baldwin AS. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923-5929. [PubMed] |
| 19. | Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2159] [Cited by in RCA: 2190] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 20. | Kreuz S, Siegmund D, Rumpf JJ, Samel D, Leverkus M, Janssen O, Häcker G, Dittrich-Breiholz O, Kracht M, Scheurich P. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J Cell Biol. 2004;166:369-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Rathore N, Matta H, Chaudhary PM. An evolutionary conserved pathway of nuclear factor-kappaB activation involving caspase-mediated cleavage and N-end rule pathway-mediated degradation of IkappaBalpha. J Biol Chem. 2004;279:39358-39365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Wu MX, Ao Z, Prasad KV, Wu R, Schlossman SF. IEX-1L, an apoptosis inhibitor involved in NF-kappaB-mediated cell survival. Science. 1998;281:998-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Islam S, Hassan F, Mu MM, Ito H, Koide N, Mori I, Yoshida T, Yokochi T. Piceatannol prevents lipopolysaccharide (LPS)-induced nitric oxide (NO) production and nuclear factor (NF)-kappaB activation by inhibiting IkappaB kinase (IKK). Microbiol Immunol. 2004;48:729-736. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1213] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 25. | Legendre F, Dudhia J, Pujol JP, Bogdanowicz P. JAK/STAT but not ERK1/ERK2 pathway mediates interleukin (IL)-6/soluble IL-6R down-regulation of Type II collagen, aggrecan core, and link protein transcription in articular chondrocytes. Association with a down-regulation of SOX9 expression. J Biol Chem. 2003;278:2903-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721-7730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002;110:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Kühnel F, Zender L, Paul Y, Tietze MK, Trautwein C, Manns M, Kubicka S. NFkappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem. 2000;275:6421-6427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Gil J, Alcamí J, Esteban M. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-kappaB. Mol Cell Biol. 1999;19:4653-4663. [PubMed] |