Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6631
Revised: June 15, 2005
Accepted: June 18, 2005
Published online: November 14, 2005
AIM: Toll-like receptor 4 (TLR4) has been shown to be important for bacterial infection, especially to lipopolysaccharide signaling. Its possible role in HBV infection is studied in the present study.
MATERIALS AND METHODS: pHBV3.6 plasmid, containing full-length HBV genome was used in the murine model of acute HBV expression by hydrodynamics in vivo transfection. TLR4 normal or mutant mouse strain was compared to investigate the possible role of TLR4 in acute HBV expression.
RESULTS: After pHBV3.6 injection, the infiltrating leukocytes expressed TLR4 were observed nearby the HBsAg-expressing hepatocytes. The HBV antigenemia as well as the replication and transcription were higher in TLR4-mutant C3H/HeJ mice than in normal C3H/HeN mice. The HBV-specific immune responses were impaired in the liver or spleen of the C3H/HeJ mice. Their inducible nitric oxide synthase (iNOS) expression on the hepatic infiltrating cells was also impaired. When adoptively transferring splenocytes from C3H/HeN mice to C3H/HeJ mice, the HBV replication was inhibited to the level as that of C3H/HeN.
CONCLUSION: These results suggest that TLR4 plays an anti-HBV role in vivo through the induction of iNOS expression and HBV-specific immune responses after HBV expression.
- Citation: Chang WW, Su IJ, Lai MD, Chang WT, Huang W, Lei HY. Toll-like receptor 4 plays an anti-HBV role in a murine model of acute hepatitis B virus expression. World J Gastroenterol 2005; 11(42): 6631-6637
- URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6631.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6631
Hepatitis B virus (HBV) is an enveloped, double-strand DNA virus, and its replication is through an RNA intermediate that requires reverse transcriptase activity[1]. HBV infection in human beings can cause chronic hepatitis and is associated with liver cirrhosis and hepatocellular carcinoma[2,3]. One-third of the global population has been infected with HBV and about 350 million people are chronic carriers of HBV[4]. HBV is non-cytopathic to hepatocytes and the hepatitis it causes is thought to be mediated by immune mechanism. However, the innate immune response to HBV infection is not fully understood.
Toll receptors are type I membrane proteins that was first identified in Drosophila and play a key role in antifungal immunity of Drosophila[5]. The mammalian homologs of Drosophila Toll protein are called Toll-like receptors (TLRs), and there are 10 human (TLR1 to TLR10) and murine TLRs (TLR 1 to TLR9 and TLR11)[5,6]. TLRs play a key role in host defense against microbial infection by regulating both innate and acquired immunity[7,8]. For example, TLR4 is the receptor of Gram-negative bacterial lipopolysaccharide (LPS). After binding, the MyD88, interleukin-1 receptor-associated kinase, and tumor necrosis factor receptor associated factor 6 are activated, and then through MAP kinases and NF-κ B transcription factors[6,9] to turn on the genes expression, which were involved in the inflammatory responses[10]. TLR4 also plays a role in viral infections. Respiratory syncytial virus (RSV) persists longer in the lung of TLR4-deficient mice than normal mice, and RSV fusion protein can activate the human monocytes through TLR4[11].
Hydrodynamics-based in vivo transfection has been recently described. With this procedure, naked DNA can be introduced and expressed significantly in liver[12,13]. This property allows investigators to develop hepatitis virus infection model in mouse[14]. Recently, a murine acute HBV expression model was generated by hydrodynamics-based injection of plasmid containing full-length HBV genome by our group[15] or others[16]. After hydrodynamic injection of pHBV3.6, including full-length HBV genome, the HBV transcript and replicative intermediate were induced in the liver whereas the HBV-antigens, HBV-DNA, and HBV-specific antibody were detected in the sera[15].
We are interested in the role of TLR4 during HBV infection. Using the murine model of acute HBV expression in this study, we reported that HBV expression-induced TLR4 expression has anti-HBV activity by upregulating the iNOS expression and HBV-specific immune response to help clearing the virus.
Breeder mice of C3H/HeN and C3H/HeJ strain were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) or Charles River Japan, Inc. (Atsugi, Japan). They were fed standard laboratory chow and water ad libitum in the animal facility. The animals were raised and cared for according to the guidelines set up by the National Science Council of the Republic of China. Eight- to twelve-week-old male mice were used in all experiments.
pHBV3.6 containing all HBV open-reading frames was provided by Dr LP Ting (Department of Microbiology and Immunology, National Yang-Ming University), p(3A)SAg that encodes HBsAg was provided by Dr CC Lu (Department of Pathology, National Cheng Kung University) and pHBV^PSX that encodes HBcAg was provided by Dr SJ Lo (Department of Microbiology and Immunology, National Yang-Ming University). pEGFP-N1 was obtained from Clontech (Palo Alto, CA, USA). All plasmids were prepared with Hi-speed Plasmid Midi Kit (Qiagen, Hilden, Germany).
The C3H/He bladder cancer cell line, MBT-2, was kindly provided by Dr MD Lai (Department of Biochemistry, National Cheng Kung University). Cells were maintained in Dulbecco’s modified Eagle medium (Gibco BRL, Grand Island, NY, USA) and 10% fetal bovine serum (HyClone, Logan, UT, USA) at 37 °C under 50 mL/L CO2. The cells were plated at a density of 3×105 cells/well in six well-culture plate. One day later, cells were transfected with 1 μg of p(3A)SAg and pHBV^PSX using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The medium was replaced with a fresh medium 8 h after transfection, and cells were used for T cell stimulation at 36 h after transfection.
Ten micrograms of plasmid, dissolved in Ringer’s solution (NaCl 0.154 mol/L, KCl 5.63 mmol/L, CaCl2 2.25 mmol/L), were injected in the mouse tail vein, within 5 to 7 s, at a 12% of mouse bodyweight (around 3.0 mL) following the hydrodynamics-based transfection protocol described previously[15].
Mouse liver tissues were embedded in OCT compound (Miles Inc., Elkhart, IN, USA) and frozen in liquid nitrogen. Four micrometer cryosections were made using cryostats (Leica CM 1800, Nussloch, Germany). For TLR4 and HBsAg double staining, sections were fixed by 3.7% formaldehyde/PBS and then firstly stained with sheep-anti-HBsAg antibody (Serotec, Oxford, UK) and FITC-conjugated donkey-anti-sheep antibody (Jackson Laboratories, West Grove, PA, USA). After washing with PBS, the sections were further stained with rat-anti-mouse TLR4 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and Rhodamine-conjugated donkey-anti-rat antibody (Jackson Laboratories, West Grove, PA, USA). The nucleuses were stained by Hoecsht 33258. For HBsAg and iNOS staining, the sections were fixed by cold acetone and endogenous peroxidase was inhibited by 3% H2O2/PBS. HBsAg and iNOS were detected with sheep polyclonal anti-HBs (Serotec, Oxford, UK) and rabbit polyclonal anti-iNOS antibodies (Chemicon, Temecula, CA, USA), respectively. Secondary antibody used was either biotinylated anti-sheep or anti-rabbit, and then incubated with avidin-peroxidase complex (Vector Laboratories, Burlingame, CA, USA). Peroxidase stain of red color was developed by aminoethyl carbazole substrate (Zymed Laboratories, San Francisco, CA, USA) and counterstained with Mayer’s hematoxylin (Merck, Darmstadt, Germany).
Total RNA was purified from mouse liver by TRI Reagent (Molecular Research Center, Inc, Cincinnati, OH, USA). The cytoplasmic DNA of mouse liver was purified as described previously by Guidotti et al[17]. Thirty micrograms of total RNA or cytoplasmic DNA isolated from 30 mg of liver tissue were run on agarose gel electrophoresis, transferred to a nylon membrane and hybridized with biotin labeled HBV specific DNA probe, which was prepared by PCR amplication with the primers as: HBV1806: 5’-CCGGAAAGCTTGAGCTCTTCAAAAAGTATGGTGCTGG-3’ ; HBV1821: 5’-CCGGAAAGCTTCTTTTTCACCTCTGCCTAATCA-3’, at 45 °C overnight. To verify the transfection efficiency, we injected pEGFP-N1 with pHBV3.6 simultaneously and detected by a specific biotin labeled DNA probe. The hybridized bands were detected by Detector AP Chemiluminescent Blotting Kit (KPL, Inc., Gaithersburg, MD, USA) and visualized by X-ray films.
Two hundred microliters of mouse serum was treated with 20 U DNase I for at least 12 h to remove retaining plasmid. After DNase I treatment, the serum DNA was purified by Viral DNA/RNA Isolation Kit (Maxim Biotech, INC., San Francisco, CA, USA). Five microliters of isolated DNA solution was used to detect the HBV DNA by PCR analysis. The preS2 region of surface antigen gene was amplified and visualized on an agarose gel as described previously[18].
The level of HBsAg and HBeAg were determined using enzyme-linked immunosorbent assay (ELISA) kits (General Biological Corp., Taiwan, ROC) following the manufacturer’s protocol.
The intrahepatic lymphocytes (IHLs) were isolated as described previously[15] with further removing the adhering cells. The IHLs or splenocytes were co-cultured with mitomycin C-treated (100 μg/mL at 37 °C for 90 min) MBT-2[19] or p(3A)SAg and pHBV^PSX transfected, MBT-2 (MBT-2-SC) at a ratio of 10:1 for 48 h The co-cultured supernatants were harvested and assayed by sandwich ELISA for mouse IFN-γ, TNF-α or IL-12 (R&D Systems, Minneapolis, MN, USA).
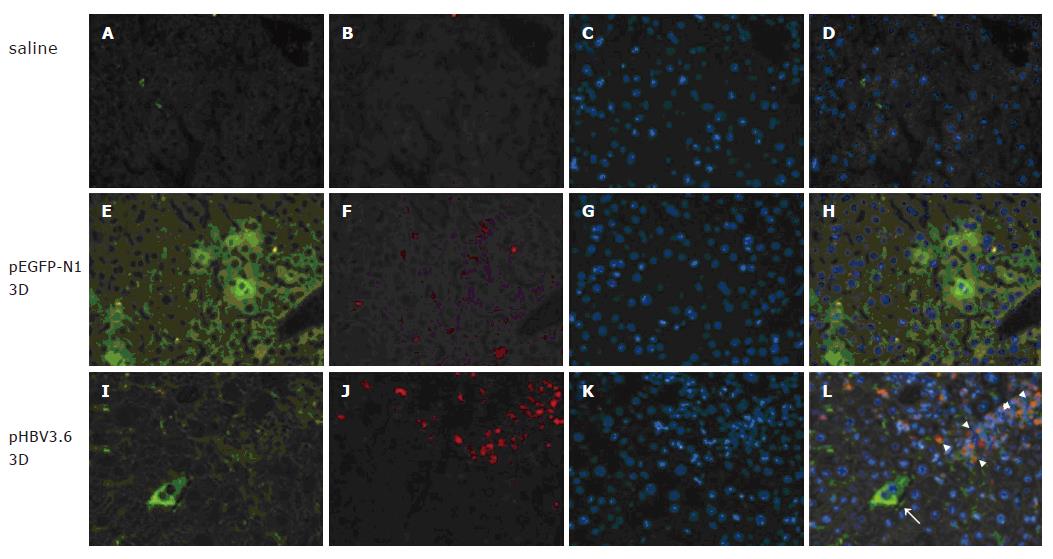
To investigate the association of TLR4 and HBV ex-pression, the plasmid pHBV3.6, containing the full-length HBV genome was administrated to C3H/HeN mice by hydrodynamics in vivo transfection. As shown in Figure 1, the TLR4 expression could be detected on infiltrating leukocytes at day 3 post-injection (Figures 1I-L). But it was not detected in the liver of naïve C3H/HeN mice (Figures 1A-D) or pEGFP-N1 injected mice (Figures 1E-H). These results indicate that TLR4 expressed on leukocytes might involve the acute HBV expression.
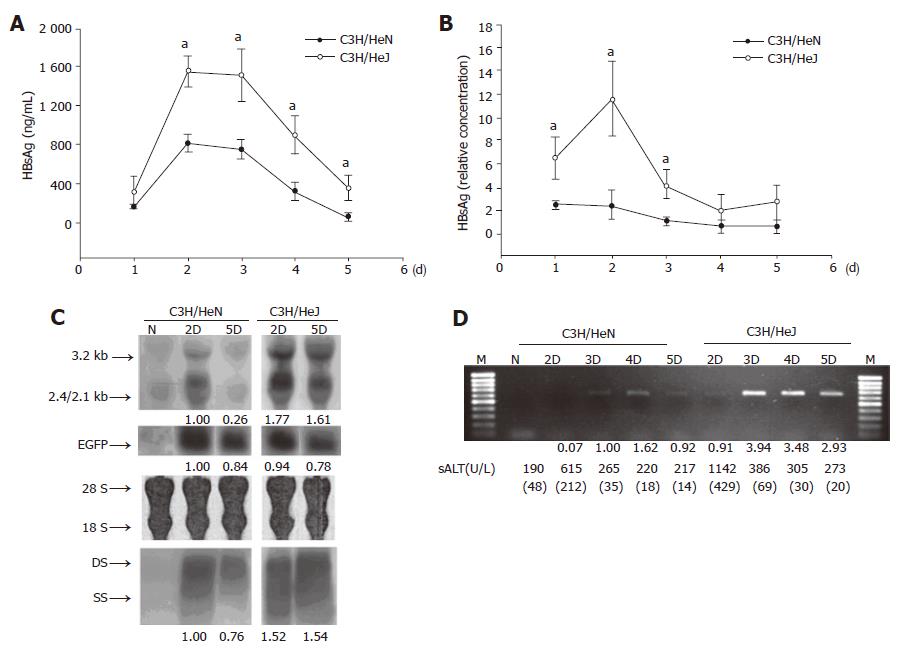
In C3H/HeJ mice, a missense mutation in the cytoplasmic domain of TLR4 impaired its ability to respond to lipopolysaccharide (LPS)[20]. To further investigate the role of TLR4 in HBV expression, we compared the response in C3H/HeN (TLR4 normal) and C3H/HeJ (TLR4 mutation) after the pHBV3.6 in vivo transfection. As shown in Figure 2, the serum HBsAg (Figure 2A) or HBeAg (Figure 2B) level was higher in C3H/HeJ than in C3H/HeN mice. By Northern and Southern blot analyses, the specific HBV transcripts or HBV replication fragments were also higher in the liver of C3H/HeJ than C3H/HeN mice under similar transfection efficiency that was verified by co-injection of pEGFP-N1 (Figure 2C). Using viral DNA isolation and PCR analysis, the serum HBV-DNA showed higher level in C3H/HeJ than in C3H/HeN mice (Figure 2D). These results suggest that TLR4 is involved in host responses to HBV replication and a mutation in C3H/HeJ impaired its ability to clear the HBV virus.
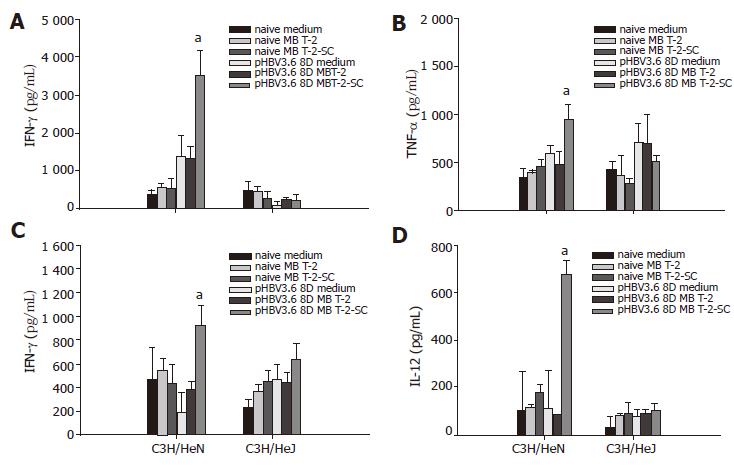
To investigate the effect of TLR4 on immune responses to HBV, intrahepatic lymphocytes (IHLs) or splenocytes from pHBV3.6-injected C3H/HeN or C3H/HeJ mice were isolated and stimulated with HBsAg- and HBcAg-expressing syngenic MBT-2 cells. The IFN-γ and TNF-α production of IHLs from C3H/HeN mice was significantly increased at 48 h after HBsAg and HBcAg stimulation. But this was not observed in IHLs from C3H/HeJ mice (Figures 3A and B). The IFN-γ and IL-12 production of splenocytes of C3H/HeN mice was also significantly increased in C3H/HeN mice, but not in C3H/HeJ mice (Figures 3C and D). These data suggest that TLR4 mutation in C3H/HeJ affects the HBV specific immune responses (IFN-γ and TNF-α production in liver or IFN-γ and IL-12 production in spleen).
It was reported that the activation of TLR4 signaling can induce the iNOS expression[21], and we also reported that iNOS plays an anti-HBV role in acute HBV expression[15]. Therefore, the iNOS expressed was compared in wild type and TLR4 mutant mice after pHBV3.6 transfection. The HBsAg-expressing hepatocytes were equivalent among C3H/HeN and C3H/HeJ mice after pHBV3.6 injection, indicating the transfection efficiency was similar in both strains of mice. But iNOS stainings were detected on the infiltrating leukocytes nearby the HBsAg-expressing hepatocytes in the liver of pHBV3.6-injected C3H/HeN (Figures 4C and D) whereas the expression of iNOS on infiltrating leukocytes was impaired in the liver of C3H/HeJ mice (Figures 4G and H). These data suggested that the mutation of TLR4 influences the induction of iNOS expression during acute HBV expression and may further affect the clearance of HBV.
To further confirm, if TLR4-expressing immune cells provide the protection role to HBV expression, the splenocytes from C3H/HeN mice were injected into C3H/HeJ mice intravenously at the day before pHBV3.6 injection. The HBsAg antigenemia was higher in C3H/HeJ than in C3H/HeN mice, but the adoptive transfer of C3H/HeN splenocytes into the C3H/HeJ mice reduced the serum HBsAg to the level similar to that in C3H/HeN mice (Figure 5A). The HBV transcription or replication in liver (Figure 5B) or HBV-DNA in sera (Figure 5C) was also reduced as well under similar transfection efficiency as verified by co-injection of pEGFP-N1. But when adoptive transfer of splenocytes from C3H/HeJ mice to C3H/HeN mice was carried out, there was no effect to HBsAg in sera (Figure 5A), HBV replication in liver (Figure 5B), or HBV-DNA in sera (Figure 5C). These results indicate that the TLR4 enhances the immune functions to help clear the HBV replication.
In this study, we investigated the role of TLR4 in a murine acute HBV expression model. The HBV antigenemia, serum HBV-DNA, as well as HBV transcription and replication in liver were higher in TLR4 mutant C3H/HeJ than TLR4 normal C3H/HeN. This is probably caused by the impaired induction of iNOS and HBV specific immune response because of TLR4 mutation in C3H/HeJ mice, and suggests that TLR4 plays an antiviral role in HBV replication. Recently, Isogawa et al[22] reported that TLR signaling which includes TLR4 could inhibit HBV replication in transgenic mice model and the mechanism might be through the induction of type I interferon. Our finding of iNOS and HBV specific immune response has increased further understanding on anti-HBV response in addition to the induction of type I interferon.
The TLRs are pattern-recognition receptors that have an important role in mammalian immunity[7,8]. At least 10 TLRs are expressed on a variety of cell types including immune cells, endothelial cells[23], cardiac myocytes[24], and intestinal epithelial cells[25]. Most TLR ligands are conserved pathogen-associated molecular patterns of microbes and the TLR signals serve as a sensor to the presence of infection[13]. TLR4 is the first identified mammalian TLR that expresses predominantly on macrophages and dendritic cells (DCs)[5]. A point mutation (His712→Pro712) in the Toll/interleukine-1 receptor domain of TLR4 gene causes the C3H/HeJ mice to become defective after LPS challenge[20,26]. TLR4 has been shown to initiate a response to the fusion protein of RSV[11]. Prolonged RSV infection was found in TLR4-deficient mice because of the impaired recruitment of natural killer cells and CD14+ cells in the lung tissue as well as the impaired IL-12 production[27]. Two vaccinia virus ORFs, A46R, and A52R, have been shown to share amino acid sequence similarity to TIR domain, and these two proteins can partially or potentially inhibit the IL-1 and TLR4 mediated NF-κB activation[28]. These reports indicate that TLR4 also has anti-virus activity in addition to its anti-bacterial function.
In our study, the IHLs nearby the HBsAg-positive hepatocytes (which represented HBV-replicating he-patocytes) expressed TLR4 post pHBV3.6 hydrodynamic transfection (Figure 1). The HBV replication was higher in TLR4 mutant C3H/HeJ than C3H/HeN mice (Figure 2). This is probably caused by the impaired specific anti-HBV immunity in liver or spleen (Figure 3). When adoptively transferring splenocytes from TLR4 normal C3H/HeN mice to TLR4 mutant C3H/HeJ mice, the anti-HBV responses could be upregulated (Figure 5). These results indicate that anti-HBV specific immune responses may be initiated by TLR4 activation. Activation of DCs by LPS through TLR4 has been found to induce IL-12 production and elicit the Th1 responses against intracellular pathogens[29]. In our study, the IL-12 production in splenocytes after HBsAg or HBcAg stimulation (Figure 3D) and IFN-γ production in IHLs (Figure 3A) was also impaired in the C3H/HeJ mice. These results correlated with the impaired function of TLR4 in the C3H/HeJ mice.
TLR4 can signal to induce iNOS expression. After TLR4 agonist stimulation, iNOS gene could be induced through MyD88-dependent (NF-κB) or -independent (IFN-β and STAT-1) pathway in murine macrophage cell line[21]. We have also reported the role of iNOS in anti-HBV response[15]. The iNOS induction in the infiltrating immune cells was impaired in C3H/HeJ mice (Figure 4). Therefore, the iNOS may also involve in the TLR4-mediated anti-HBV responses. In conclusion, the present study reports that TLR4 plays an anti-HBV role in acute HBV expression through induction of iNOS expression and specific anti-HBV immune responses.
We thank M. Theron for editorial assistance.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Nassal M, Schaller H. Hepatitis B virus replication. Trends Microbiol. 1993;1:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Arbuthnot P, Kew M. Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol. 2001;82:77-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Feitelson MA. Hepatitis B virus in hepatocarcinogenesis. J Cell Physiol. 1999;181:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 5. | Lien E, Ingalls RR. Toll-like receptors. Crit Care Med. 2002;30:S1-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 727] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 7. | Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3474] [Cited by in RCA: 3509] [Article Influence: 146.2] [Reference Citation Analysis (0)] |
| 8. | Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2272] [Cited by in RCA: 2242] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 9. | Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 1005] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 10. | Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4113] [Cited by in RCA: 4135] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 11. | Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1210] [Cited by in RCA: 1204] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 12. | Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1387] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 13. | Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 747] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 14. | Chang J, Sigal LJ, Lerro A, Taylor J. Replication of the human hepatitis delta virus genome Is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. J Virol. 2001;75:3469-3473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Chang WW, Su IJ, Lai MD, Chang WT, Huang W, Lei HY. The role of inducible nitric oxide synthase in a murine acute hepatitis B virus (HBV) infection model induced by hydrodynamics-based in vivo transfection of HBV-DNA. J Hepatol. 2003;39:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Yang PL, Althage A, Chung J, Chisari FV. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci USA. 2002;99:13825-13830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 324] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Guidotti LG, Matzke B, Schaller H, Chisari FV. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158-6169. [PubMed] |
| 18. | Fan YF, Lu CC, Chen WC, Yao WJ, Wang HC, Chang TT, Lei HY, Shiau AL, Su IJ. Prevalence and significance of hepatitis B virus (HBV) pre-S mutants in serum and liver at different replicative stages of chronic HBV infection. Hepatology. 2001;33:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Eto M, Harada M, Tamada K, Tokuda N, Koikawa Y, Nakamura M, Nomoto K, Naito S. Antitumor activity of interleukin-12 against murine bladder cancer. J Urol. 2000;163:1549-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5767] [Cited by in RCA: 5778] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 21. | Schilling D, Thomas K, Nixdorff K, Vogel SN, Fenton MJ. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J Immunol. 2002;169:5874-5880. [RCA] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269-7272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 360] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 23. | Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. 2001;166:2018-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 355] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197-5203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 198] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 566] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 26. | Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749-3752. [PubMed] |
| 27. | Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730-10737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 367] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Bowie A, Kiss-Toth E, Symons JA, Smith GL, Dower SK, O'Neill LA. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc Natl Acad Sci U S A. 2000;97:10162-10167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 363] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984-4989. [RCA] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 581] [Article Influence: 27.7] [Reference Citation Analysis (0)] |